41 dry cell battery diagram
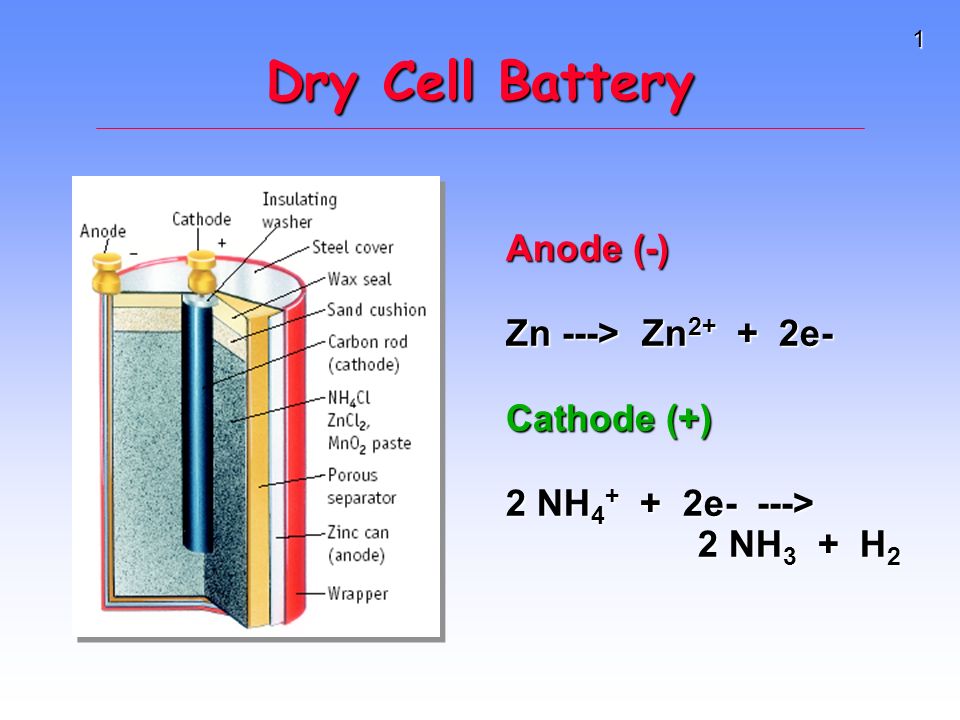
Dry Cell : It is a primary cell based on Leclanche cell invented by G. Leclanche in 1868. In a primary cell, the electrode reactions cannot be reversed by an external source of electrical energy. In this cell, the cell reaction takes place only once i.e., this cell is not rechargeable. It is.generally used intorched, transistors, radios ... A dry cell battery is a type of chemical battery that uses an electrolyte, which is in the immobilized state. The electrolyte in this cell battery contains very little moisture to allow the passage of current through it. This ScienceStruck post provides the history, definition, composition, uses, and recycling process of the dry cell battery.
As we have just seen, cells require a constant supply of energy to generate and maintain the biological order that keeps them alive. This energy is derived from the chemical bond energy in food molecules, which thereby serve as fuel for cells.. Sugars are particularly important fuel molecules, and they are oxidized in small steps to carbon dioxide (CO 2) and water (Figure 2-69).

Dry cell battery diagram
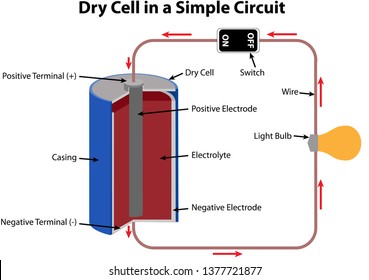
Draw circuit diagram showing a dry cell connected to a bulb through a switch, a positive and negative terminal of the cell and the direction of flow of electric current and conventional current. ... Verified. 121.8k+ views. Hint: Draw the circuit diagram of the bulb, battery and key connected in series. The electrons move from the negative ... The dry cell battery is one of the most commonly used types, including AA, 9-volt, and watch batteries. Dry cell batteries are different from wet cells because their electrolytes are contained in a low-moisture paste, while a wet cell has electrolytes contained in a liquid, hence the difference in names. dry cell, energy is made from a paste of electrolytes. Most of our batteries are dry cells. D ry cells are safer than wet cells. They are also easier to use. 4. A dry cell has 3 parts: the cathode (+), the anode (-), and the electrolyte. The cathode is the tip of the battery with the + sign. The anode is the tip of the battery with the - sign.
Dry cell battery diagram. 1. Use circuit symbols to construct schematic diagrams for the following circuits: a. A single cell, light bulb and switch are placed together in a circuit such that the switch can be opened and closed to turn the light bulb on. See Answer. b. A three-pack of D-cells is placed in a circuit to power a flashlight bulb. Find Crosssection Cutaway Diagram Dry Cell Battery stock images in HD and millions of other royalty-free stock photos, illustrations and vectors in the Shutterstock collection. Thousands of new, high-quality pictures added every day. A dry cell is the simplest form of electricity-producing source. A number of cells combined cells together forms a battery. The lead-acid or nickel-cadmium battery is the advanced version of dry cell. This cell was first invented by French engineer Georges Leclanche in the year 1866. Find Parts Dry Cell Battery Vector Diagram stock images in HD and millions of other royalty-free stock photos, illustrations and vectors in the Shutterstock collection. Thousands of new, high-quality pictures added every day.
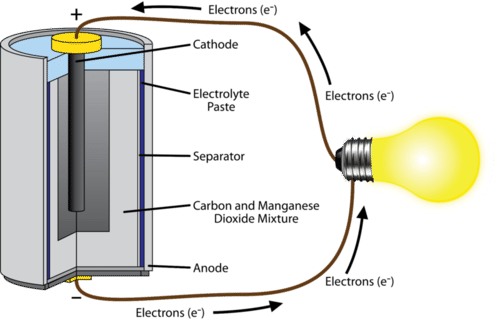
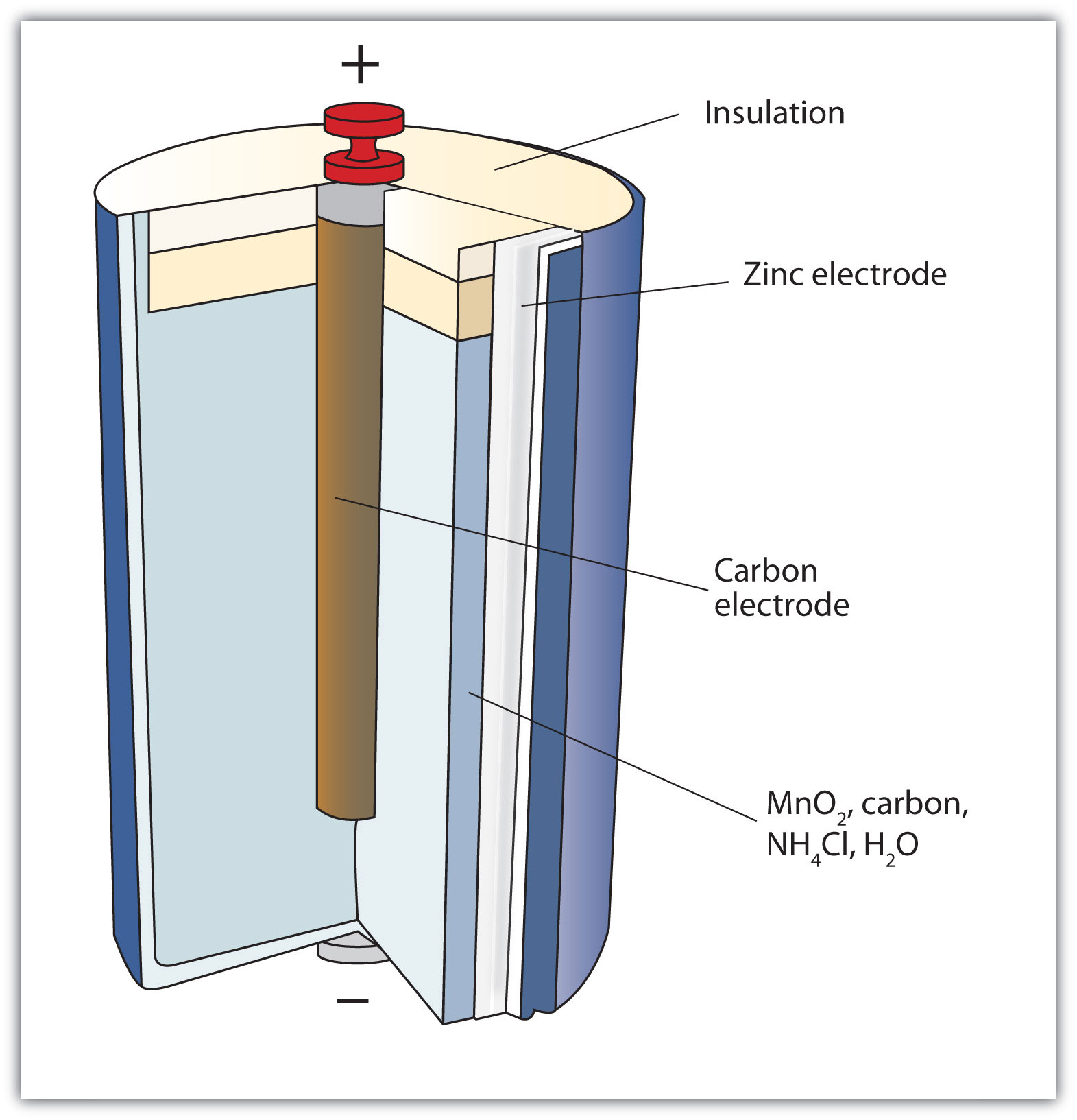
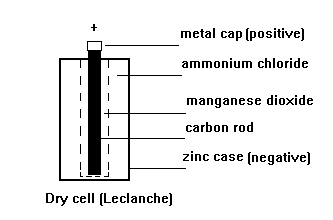
The zinc-carbon dry cell (or battery), shown in a cutaway diagram, is a modern version of the Leclanché cell. The vanadium redox battery (VRB), also known as the vanadium flow battery (VFB) or vanadium redox flow battery (VRFB), is a type of rechargeable flow battery.It employs vanadium ions as charge carriers. The battery uses vanadium's ability to exist in solution in four different oxidation states to make a battery with a single electroactive element instead of two. Leclanche Cell Diagram & Working. May 16, 2020. May 14, 2020 by Electricalvoice. The Leclanche cell is a battery which is named after the French scientist Georges Leclanché who invented it in 1866. The Leclanche cell e.m.f. is 1.5 volt. The application of the Leclanche cell was in electric bells, signalling, and telegraphy. Contents show. Battery Cell Diagram. Here are a number of highest rated Battery Cell Diagram pictures on internet. We identified it from trustworthy source. Its submitted by presidency in the best field. We acknowledge this nice of Battery Cell Diagram graphic could possibly be the most trending topic as soon as we share it in google pro or facebook.
This type of battery offers high performance, featuring high voltage and reliability, and a maximum amount of energy per volume that can be as high as ten times that of manganese dry batteries. Its electrolyte contains no water, allowing for use at low temperature. A dry cell is a type of electric battery, commonly used for portable electrical devices.It was developed in 1886 by the German scientist Carl Gassner, after development of wet zinc-carbon batteries by Georges Leclanché in 1866. The modern version was developed by Japanese Sakizō Yai in 1887. Battery Tester Project Using LM3914 IC. This objective of this project is to design and build a battery tester that is able to test various types of dry cell and rechargeable battery with a voltage of less than 2V. Configured as a bar graph battery level indicator, the LM3914 IC from National Semiconductor senses the voltage levels of the ... Transcribed image text: Consider a system consisting of a dry cell battery, buzzer, sound receivers, and their surroundings. Below is a possible G/R energy diagram showing the flow of energy through the system during the time period of time when the dry cell battery and buzzer have already reached their constant operating temperatures and the buzzer mechanism is no longer speeding up.
Which of the following is a correct definition of the term armature? A. A small lever in a relay switch B. A circuit diagram C. A type of resistant wire D. A type of dry cell battery
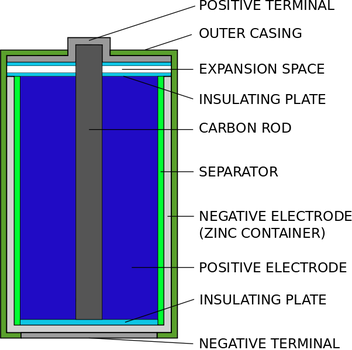
Anatomy of a Battery. Take a look at any battery, and you'll notice that it has two terminals. One terminal is marked (+), or positive, while the other is marked (-), or negative. In normal flashlight batteries, like AA, C or D cell, the terminals are located on the ends. On a 9-volt or car battery, however, the terminals are situated next to ...
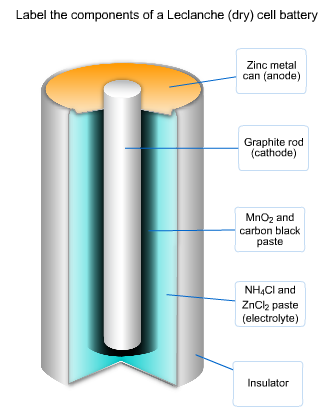
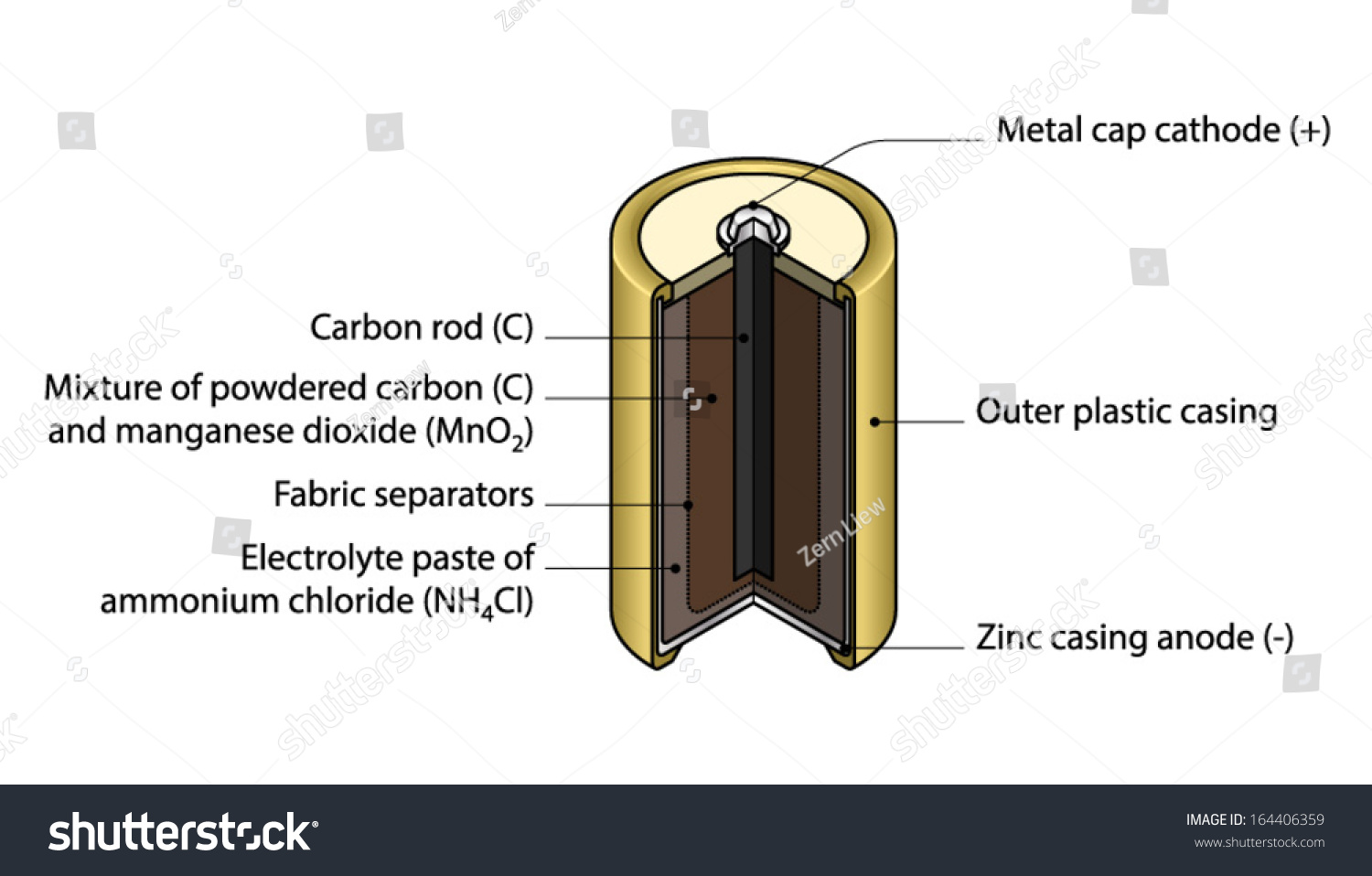
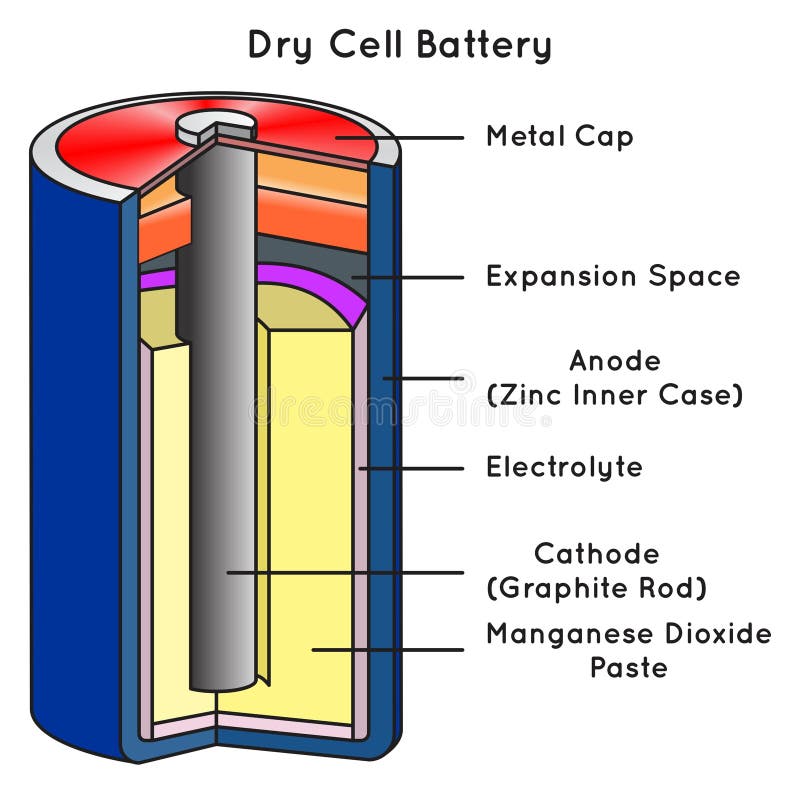
The labelled diagram for this dry cell is shown below: Note: A dry cell is basically of 2 types: primary and secondary. A primary dry cell is the one in which the electrochemical reactions consume all the chemical reagents, thus fail to produce electricity and so are neither reusable nor rechargeable. For example: zinc-carbon cell, lithium cell ...
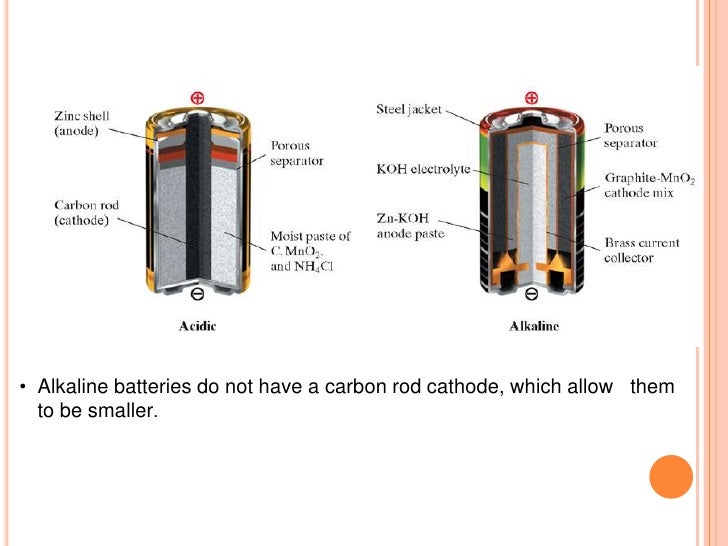
Lead-acid batteries did not achieve the safety and portability of the dry cell until the development of the gel battery. A common dry cell is the zinc-carbon battery, sometimes called the dry Leclanché cell, with a nominal voltage of 1.5 volts, the same as the alkaline battery (since both use the same zinc-manganese dioxide combination).
Bluetooth Circuits A Bluetooth circuit is the central part of a Bluetooth and contains components such as the integrated circuit, capacitors, and power source. The course supports wired-in audio, wireless stereo, Bluetooth module, and many more. We have professional standard reviewers that ensure strict quality control measures in every production process. We leverage advanced …
Dry Cell Battery Structure Diagram. Dry cell battery structure diagram including parts components electrical circuit anode cathode carbon electrode mixture jacket cup metal cover bottom separator sealant barrier film simple easy for science education. Image Editor Save Comp.
5.2.2012 · Circuit Diagram #1. Parts List. The following parts will be required for making the above explained circuit. ... the charger i got has an output of 12volts 6Amps will that charge my dry-cell battery with 200ahs capacity? If yes, ... 0-25% battery is in the charger using a red LED.25-50% using a blue LED ...
Trojan Battery Company has been manufacturing deep-cycle, flooded batteries for more than three generations. Our experience has shown that the key factor to achieving optimum performance and long battery life is to follow a regular care and maintenance program.
Alkaline Dry Cell. Here are a number of highest rated Alkaline Dry Cell pictures on internet. We identified it from obedient source. Its submitted by government in the best field. We put up with this kind of Alkaline Dry Cell graphic could possibly be the most trending topic as soon as we share it in google pro or facebook.
24.2.2012 · Working Principle of Battery. A battery works on the oxidation and reduction reaction of an electrolyte with metals. When two dissimilar metallic substances, called electrode, are placed in a diluted electrolyte, oxidation and reduction reaction take place in the electrodes respectively depending upon the electron affinity of the metal of the electrodes.
A common dry-cell battery is the zinc-carbon battery, which uses a cell that is sometimes called the Leclanché cell. The cell is made up of an outer zinc container, which acts as the anode. The cathode is a central carbon rod, surrounded by a mixture of carbon and manganese(IV) dioxide (MnO 2).
The reactants in these batteries are consumed after a certain period of time, rendering them dead. A primary battery cannot be used once the chemicals inside it are exhausted. An example of a primary battery is the dry cell - the household battery that commonly used to power TV remotes, clocks, and other devices.
This 1.5 volt "Long Life" Dry Cell is an exact replacement for the original No. 6 in terms of A-Hr capacity and run time. Folks have loved it because it is renewable. Like the majority of our other No. 6 units, the Model 1900L is protected against accidental short circuits and battery mis-installations (which happens quite frequently).
A battery is a device that creates electrical energy by means of chemical reactions.There are two types of batteries: wet cell and dry cell. A wet cell battery operates by means of a liquid electrolyte solution, while in a dry cell battery the solution is in the form of a paste. Some wet cells can be recharged, while others are only good for a shorter period of time.
A dry cell is one type of electric battery, which is generally used for the home and portable electronic devices. A battery is a device that consists of one or more electrochemical cells, which converts chemical energy into electrical energy. A dry cell is one of the electrochemical cells, developed by the "German scientists Carl Gassner ...
dry cell, energy is made from a paste of electrolytes. Most of our batteries are dry cells. D ry cells are safer than wet cells. They are also easier to use. 4. A dry cell has 3 parts: the cathode (+), the anode (-), and the electrolyte. The cathode is the tip of the battery with the + sign. The anode is the tip of the battery with the - sign.
The dry cell battery is one of the most commonly used types, including AA, 9-volt, and watch batteries. Dry cell batteries are different from wet cells because their electrolytes are contained in a low-moisture paste, while a wet cell has electrolytes contained in a liquid, hence the difference in names.
Draw circuit diagram showing a dry cell connected to a bulb through a switch, a positive and negative terminal of the cell and the direction of flow of electric current and conventional current. ... Verified. 121.8k+ views. Hint: Draw the circuit diagram of the bulb, battery and key connected in series. The electrons move from the negative ...












![PDF] ZINC-CARBON BATTERIES ( Leclanche Ì and Zinc Chloride ...](https://d3i71xaburhd42.cloudfront.net/14cb158c71abb555ee129fe6c06baa28be7870f4/5-Figure2-1.png)








Comments
Post a Comment