43 ne2+ molecular orbital diagram
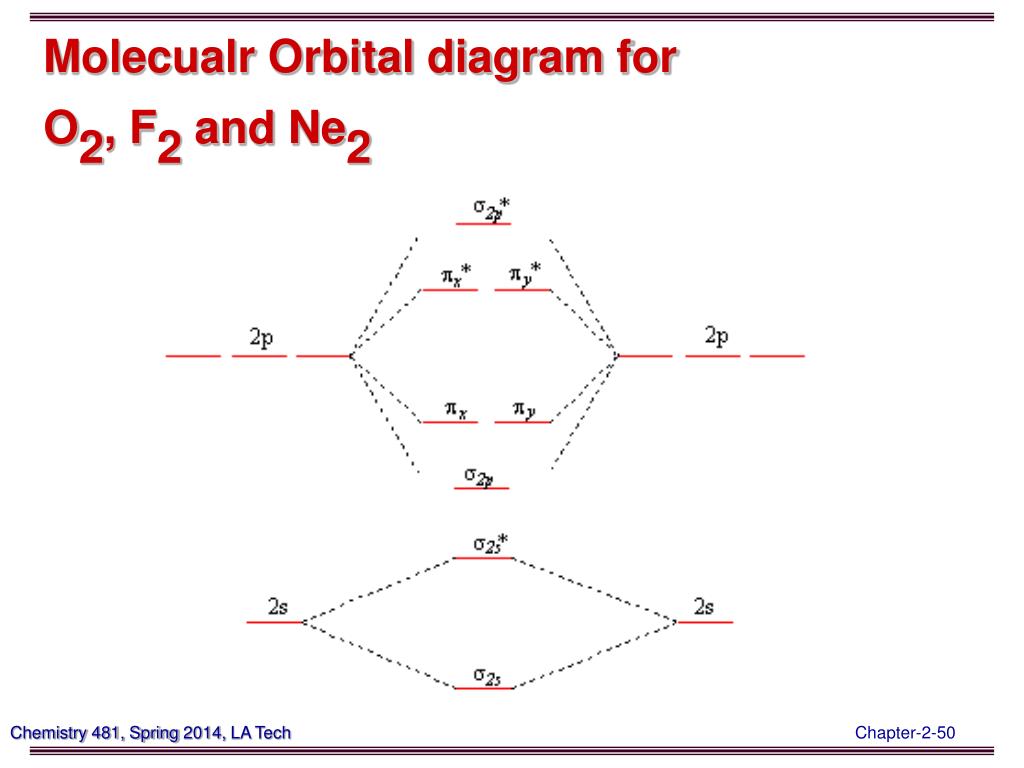
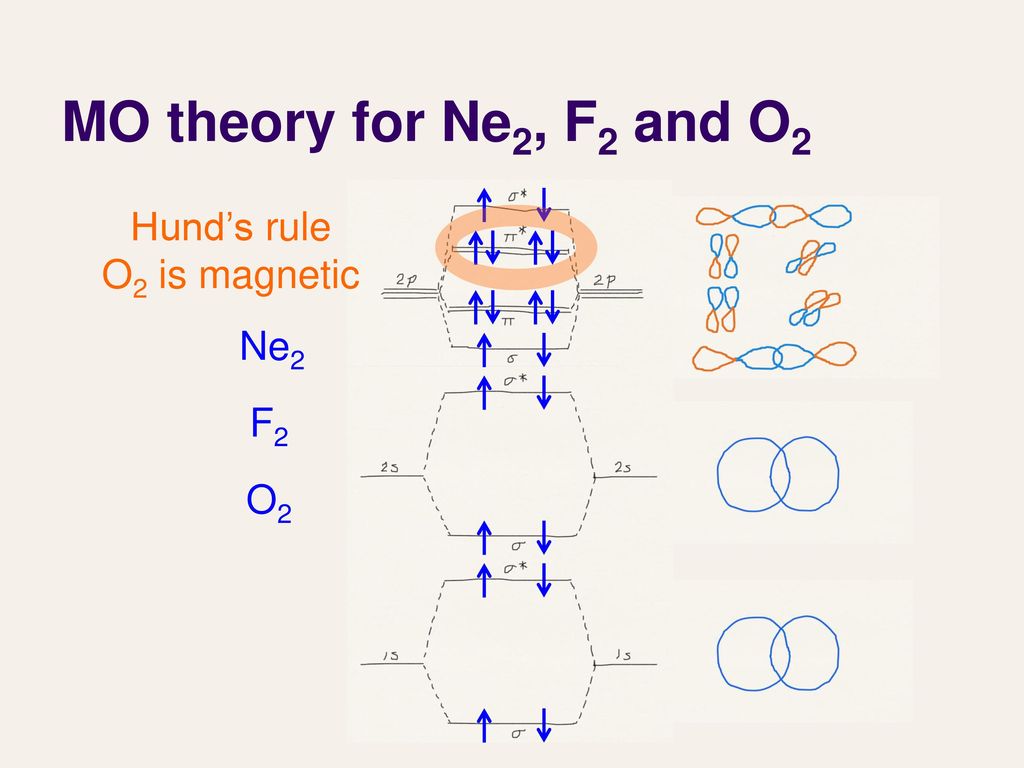
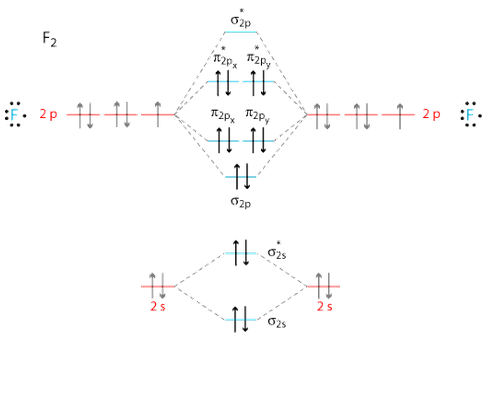
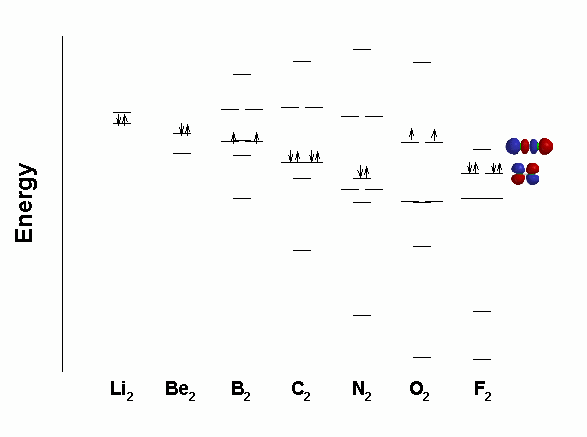
0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d... Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
OneClass: ne2+ molecular orbital diagram Chemistry 1 answer 0 watching 144 views crimsonsalmon160 Lv1 11 Dec 2019 ne2+ molecular orbital diagram Answer + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. John Edward Cayas Lv10 21 Mar 2021 Unlock all answers Get 1 free homework help answer.
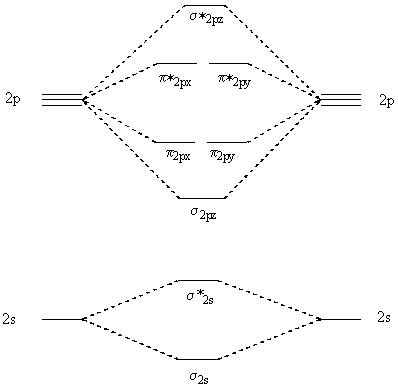
Ne2+ molecular orbital diagram
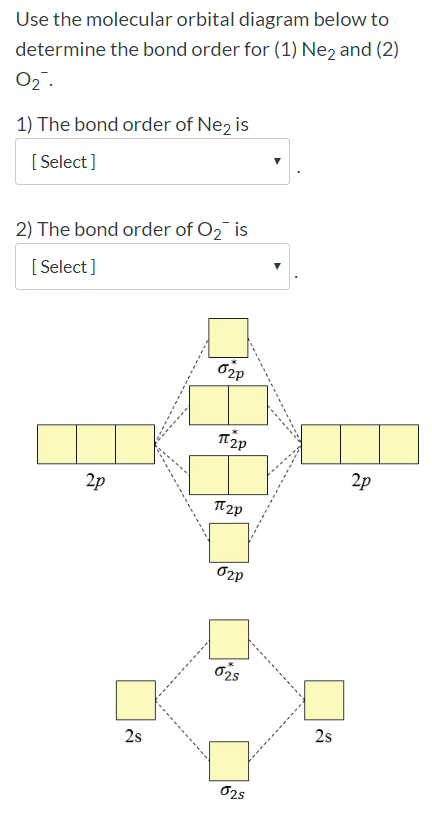
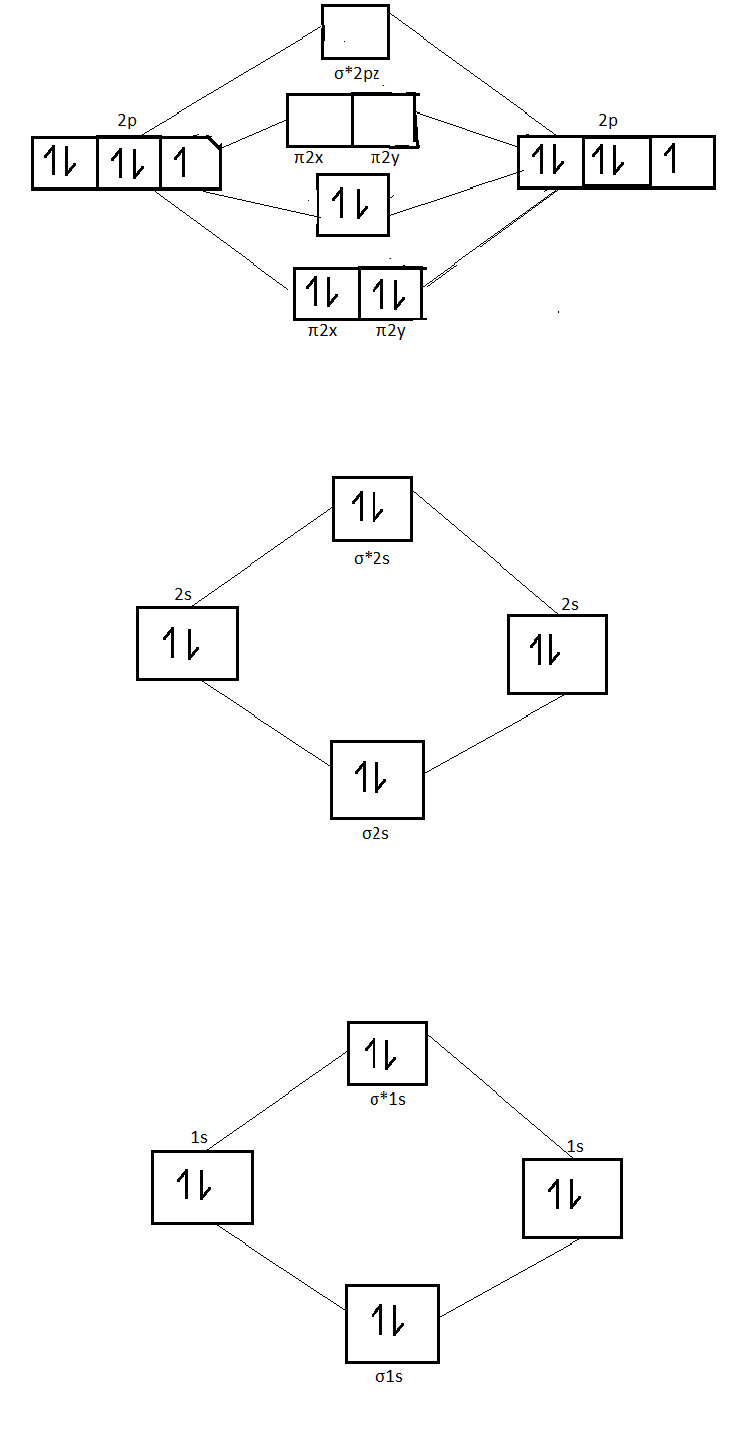
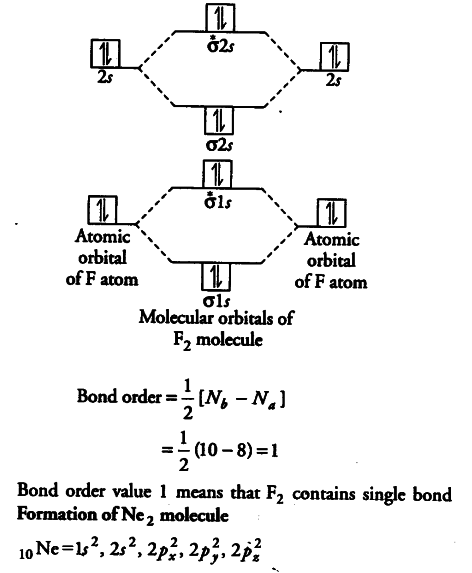
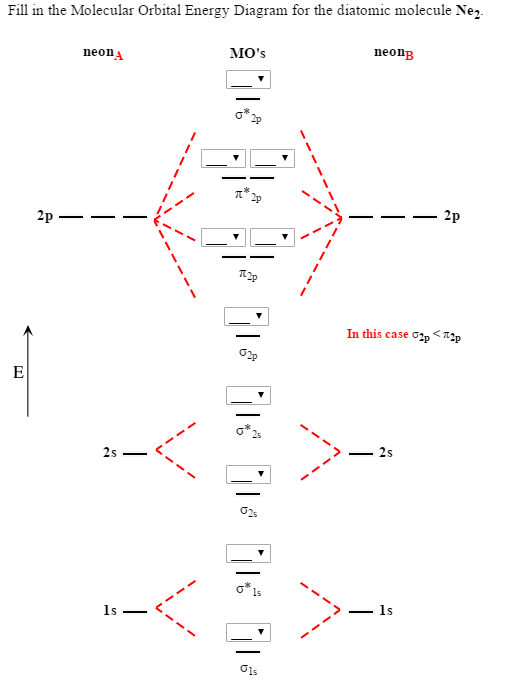
On the basis of molecular orbital diagram, explain. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window. Problem: Determine the bond order from the molecular orbital diagram of Ne2. Does the bond order calculated agree with what you would draw for Lewis ...1 answer · Top answer: We are asked to determine the bond order from the molecular orbital diagram of Ne2 and to check whether the calculated bond order agrees with the ... Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π*2Py²σ*2Pz² Hence the bond order of Ne2 according to M.O.T is = (Nb-Na)/2 = (10-10)/2 (since,both bonding orbitals and non-bonding orbital contains 10 electrons) =0 Hence, no bond is possible between 2 Ne atom. Therefore, formation of this molecule is not possible.
Ne2+ molecular orbital diagram. Free NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure solved by expert teachers from latest edition books and as per NCERT (CBSE) guidelines.Class 11 Chemistry Chemical Bonding and Molecular Structure NCERT Solutions and Extra Questions with Solutions to help you to revise complete Syllabus and Score More marks. Problem 1. After reading the theory part draw the MO diagrams for the following diatomic omonuclear molecules: H2, B2, C2, N2, O2, Ne2, F2.13 pages on Molecular Orbital Diagram For Ne2. Molecular orbital theory: Atomic orbitals (AO). Chapter 9 Energy diagrams / energy levels are often used to . theory, Ne2 doesn't exist. Ne2. Ignoring weak clusters held together by Van der Waals interactions, the reason that Ne2 does not form a stable covalent molecule can be. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or.
For Ne 2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 schematron.org each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order% (1). Academia.edu is a platform for academics to share research papers. For Ne 2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. Give each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order. Rationalize the trend in bond order in terms of bond strength. 3 Mar 2018 — Molecular orbital diagram of · Neon atom has 10 electrons and its electronic configuration is . When molecule is considered, it has two neon ...2 answers · 20 votes: Bond order of Ne2 = (10-10) =0 So Ne2 is unstable ;Ne2 cannot exist
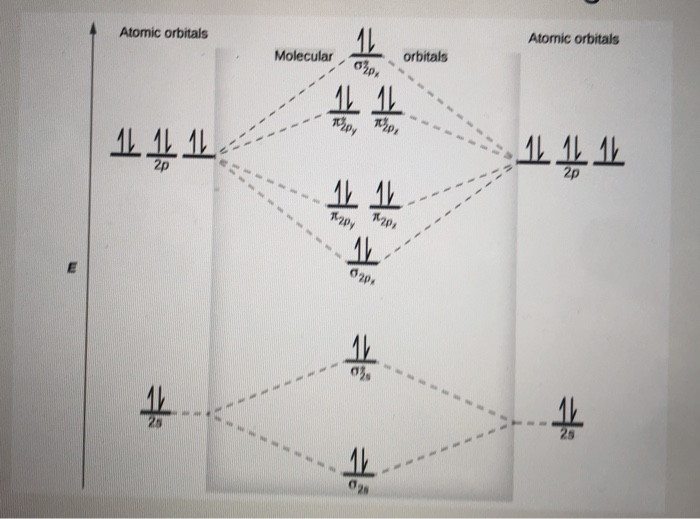
Draw out the molecular orbital diagram for Ne2, starting with the 2s atomic orbitals and label each molecular orbital with the appropriate notation as done ...4 answers · Top answer: So here we're looking at the molecular orbital theory to describe bonding. So the first example, ... Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2, a single bond and Ne2, no bond. · Solution.1 answer · Top answer: Formation of N2 molecule: Electronic Configuration, σ 1s^2<σ *1s^2<σ 2s^2<σ *2s^2<[pi 2px^2 = pi 2px^2]<<σ 2pz^2 Bond order = (Nb - Na)/2 = (10 - ... There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start... Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Academia.edu is a platform for academics to share research papers.
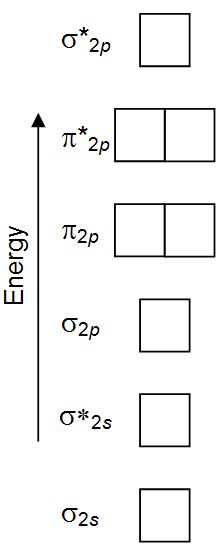
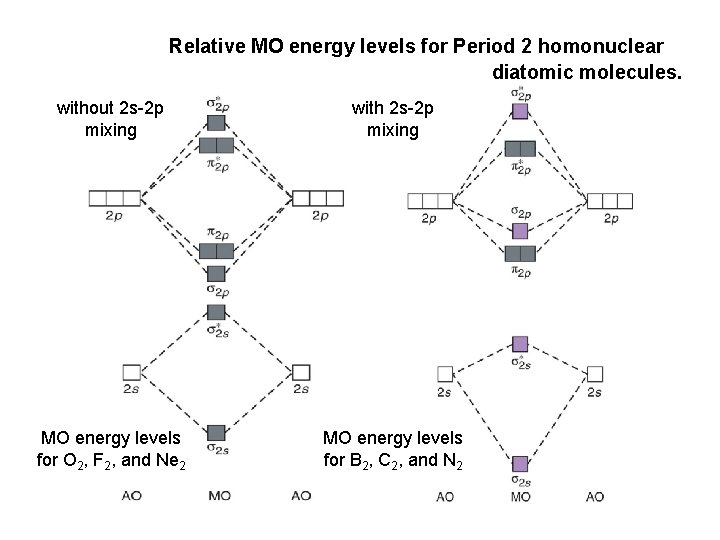
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
This is because, according to molecular orbital theory , it has fewer electrons in bonding orbitals. The diagram above is the molecular.N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the energy than the atomic and form.
5.7 a. The energy level diagram for NO is on the right. The odd electron is in a π2p* orbital. b ...29 pages
26 Jan 2020 — With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). chemical bonding ...1 answer · Top answer: Ne2 (20) = σ1s2 σ*1s2, σ2s2 σ*2s2, σpx2 π 2py2 2π* 2py2π*2py2 2pz2 σ* 2px2 B.O = 1/2 (10 - 10) = 0 Ne2 cannot exist because its bond order is zero.
Molecular Orbital Diagram Ne2 As the bond order value for molecule is zero, it is unstable and cannot exist. Molecular orbital mo diagram of n2 molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you sigma2s 2 sigma2s 2 pi2p 4 mo diagram for n2 molecular orbital there are two mo.
Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π*2Py²σ*2Pz² Hence the bond order of Ne2 according to M.O.T is = (Nb-Na)/2 = (10-10)/2 (since,both bonding orbitals and non-bonding orbital contains 10 electrons) =0 Hence, no bond is possible between 2 Ne atom. Therefore, formation of this molecule is not possible.
Problem: Determine the bond order from the molecular orbital diagram of Ne2. Does the bond order calculated agree with what you would draw for Lewis ...1 answer · Top answer: We are asked to determine the bond order from the molecular orbital diagram of Ne2 and to check whether the calculated bond order agrees with the ...
On the basis of molecular orbital diagram, explain. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window.























Comments
Post a Comment