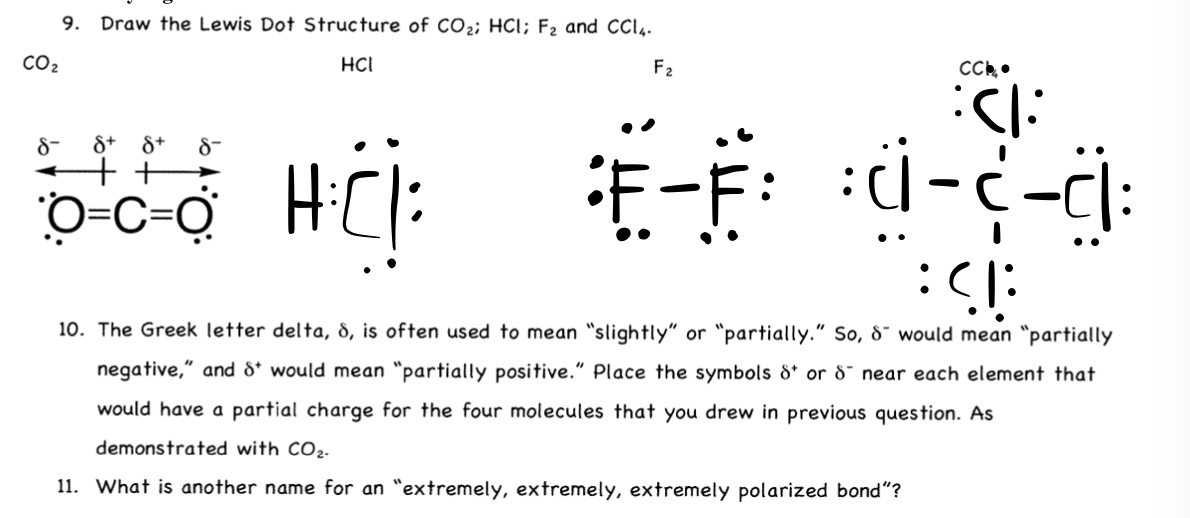
40 dot diagram for co2
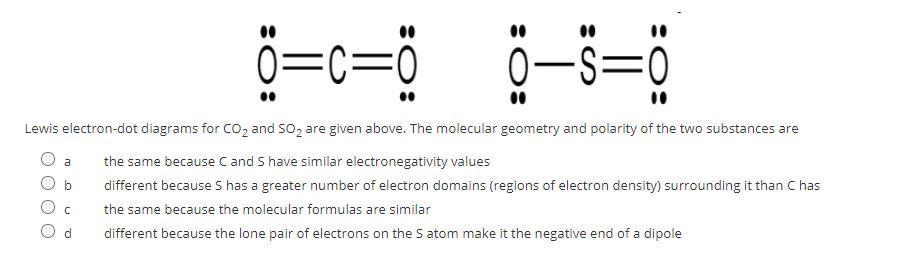
AP Chem Unit 2 Flashcards - Quizlet lewis electron dot diagrams for CO2 and SO2 are given above. The molecular geometries and polarity of the two substances are. different because the lone pair of electrons on the S atom make it the negative end of a dipole. Lewis Dot Structure of CO2 (Carbon DiOxide) - YouTube I quickly take you through how to draw the Lewis Structure of CO2 (Carbon DiOxide). I also go over hybridization, shape and bond angles.
What is the dot diagram for CO2? 2022 - Question & Answers What is the dot diagram for CO2? CO2 Lewis Structure Setup So carbon is shown with four dots around it. Oxygen needs just two bonds, represented as the lone dots to the left and right of the O atoms. The pairs of dots above and below the O's won't bond. The first thing about the CO2 Lewis structure is to put carbon in the center. admin

Dot diagram for co2
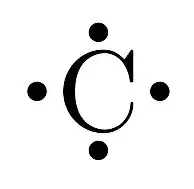
What would be the electron dot structure of carbon dioxide ... Electron dot structures or Lewis dot formula - It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. Electron dot structures of carbon dioxide. The carbon is the central atom of this molecule. Oxygen atom contains 6 valence electrons which form 2 lone pairs. CO2 Lewis Structure (2021 UPDATED) All You Need To Know 6 Steps on How to Draw CO2's Lewis Structure Calculate the total valence electrons found in a molecule. Carbon Valence Electron=4 Oxygen Valence electrons: 6*2 = 12 Total number of valence electrons = 16 Find the central atom, which is usually the one with the highest bonding sites, is the Carbon atom. Draw four dots around it. What is the Lewis dot diagram for carbon dioxide? - Quora Answer: There. That said, you could have found the exact same picture if you took 10 seconds to google "Lewis dot diagram, CO2. Sometimes I wonder.
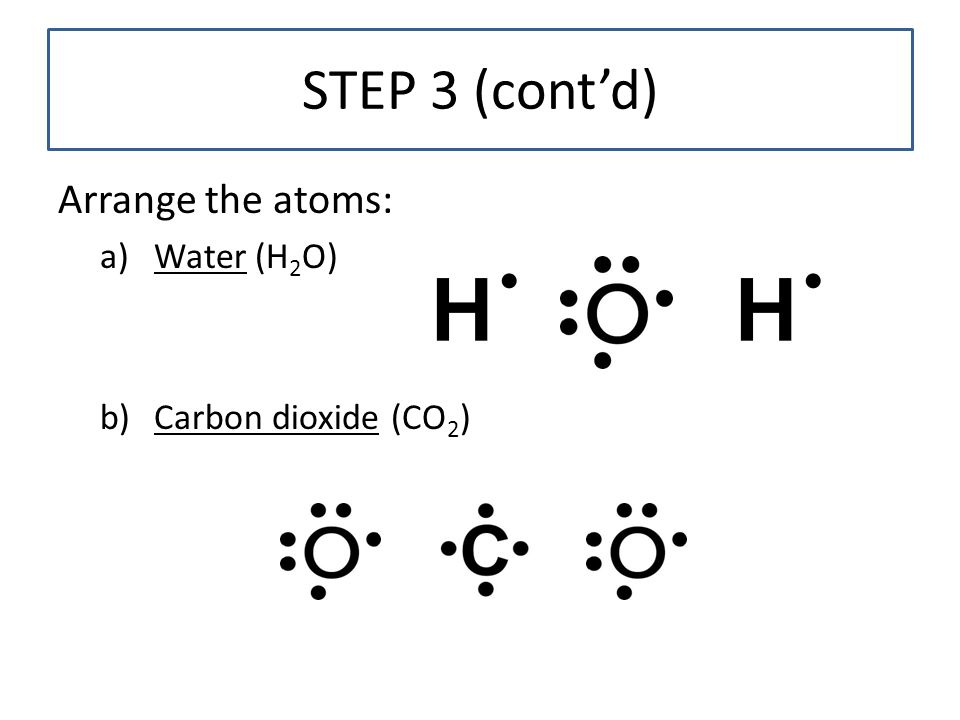
Dot diagram for co2. What is the correct electron dot formula for carbon dioxide? Carbon (C) is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structure. The Lewis structure for CO2 has a total of 16 valence electrons. In order to complete the octets for all of the atoms in the structure you will need to form two double bonds. Draw Lewis dot diagram for the following. Carbon dioxide ... Draw Lewis dot diagram for the following. Carbon dioxide (CO2) Maharashtra State Board HSC Science (Electronics) 11th. Textbook Solutions 6926. Important Solutions 17. Question Bank Solutions 4571. Concept Notes & Videos 334. Syllabus. Advertisement Remove all ads. Draw Lewis dot diagram for the following. ... How to draw CO2 Lewis Structure? - Science Education and ... To sketch the CO2 Lewis structure by following these instructions: Step-1: CO2 Lewis dot Structure by counting valence electrons on the carbon atom. Step-2: Lewis Structure of CO2 for counting valence electrons around the terminal oxygen atoms. Step-3: Lewis dot Structure for CO2 generated from step-1 and step-2. What is the electron dot diagram for CO2? - Answers An electron dot diagram can show you that the symbols for an element surrounded by dots. Each dot stands for one valence electron. ... What is the electron dot diagram for CO2?
CO2 Lewis Structure - Lewis Dot Structure | Chem Helps CO2 Lewis Dot Structure When drawing the Lewis structure of the carbon dioxide molecule, the carbon and an unpaired electron of oxygen share with each other. As a result, a single covalent bond between carbon and oxygen occurs. However, in this case, carbon and oxygen cannot complete the octet. Lewis electron dot structure of co2 - YouTube For more info regarding Lewis structures please see: ... Silicon tetrachloride (SiCl4) lewis dot structure ... “Lewis diagram describes the chemical bonding of atoms within a molecule”. Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. Let’s see how to draw this step by step-Follow some steps for drawing the lewis dot structure of SiCl4. 1. Count total valence ... CO2 (Carbon Dioxide) Lewis Dot Structure - Science Trends The Lewis Dot Structure for carbon dioxide can be represented like this:. o=C=o. But what exactly does this mean? What is a Lewis Dot Structure, and what do the symbols in carbon dioxide's structure represent?Let's go over the Lewis structure and find out how to interpret this representation of carbon dioxide.
Dot and cross diagrams for covalent bonding - Bonding ... We can use dot and cross diagrams to show how a pair of electrons forms a covalent bond. Here is the dot and cross diagram for oxygen (O2), a diatomic molecule. Notice the lone pairs of electrons... How To Draw A Lewis Dot Structure For Co2 ... What Is The Lewis Diagram For Co2? The Lewis structure of carbon dioxide (CO) consists of two oxygen atoms and one carbon atom. The carbon atom in the CO has two double bonds. The valence shells of carbon atoms and oxygen atoms each contain two lone pairs. Linearity is the characteristic of CO. What Is Co2 Dot Structure? Support Equipment For PCP/HPA/CO2 - Airguns & Guns Forum Support Equipment For PCP/HPA/CO2 (Moderator: vigilandy) ... DOT Hydro Testing Locations by State. Started by TOM aka critter99 « 1 2 » 22 Replies 14510 Views February 16, 2020, 08:57:27 PM by wahoowad: Benjamin Pump Diagram. Started by longislandhunter. 4 Replies 23728 Views July 13, 2018, 04:31:36 AM by vigilandy: SAFETY NOTICE: Molecular Seive … PDF Co2 dot and cross diagram - democratum.com Co2 dot and cross diagram Covalent Substances (Section 1g) 1.38 describe the formation of a covalent bond by the sharing of a pair of electrons between two atoms A covalent bond is a bond formed between atoms by sharing electrons (one each) with other atoms. This leaves them stable, as they now have a full outer shell.
The Lewis Dot Structure for CO2 - MakeTheBrainHappy The Lewis Dot Structure for CO2. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for CO2. You could alternatively also draw the structure by including two dots for every bond. That would mean that you would have a total of eight dots around the carbon, thereby filling its octet. The octets of both of the oxygen atoms are also ...
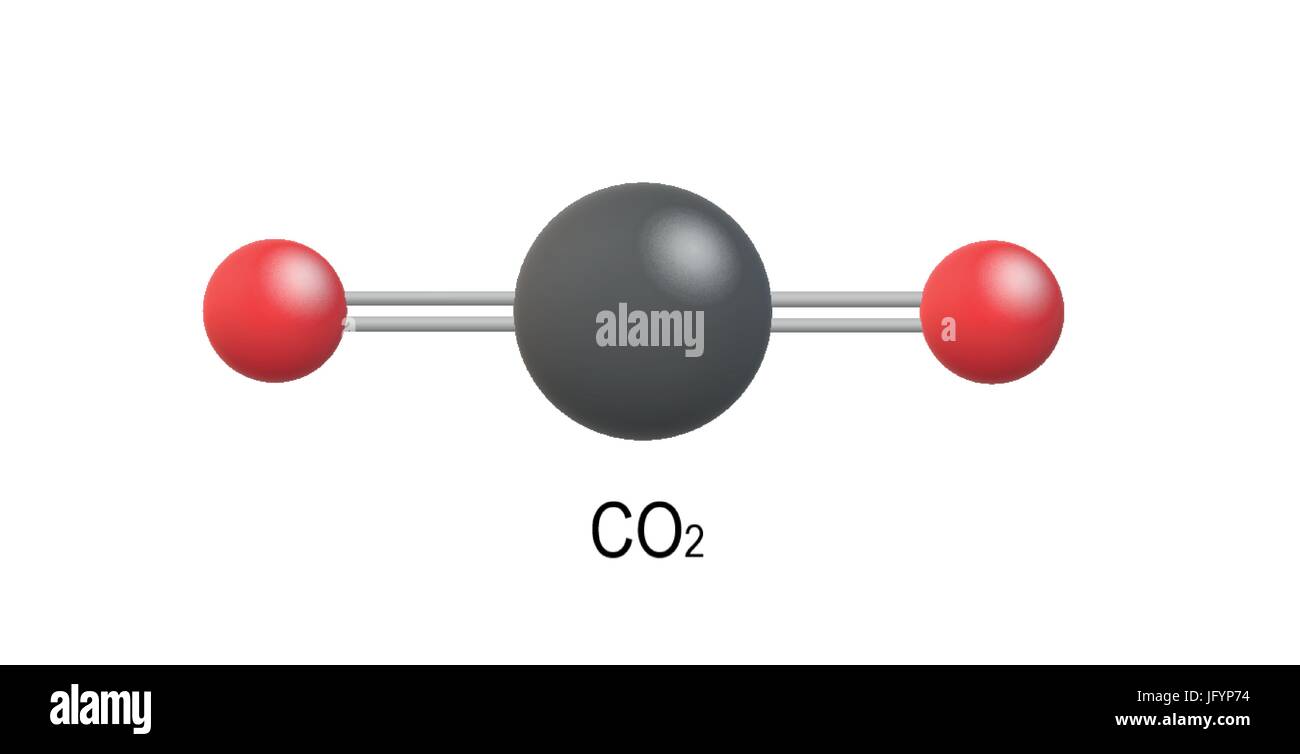
CO2 (Carbon dioxide) Lewis Structure and Shape Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial.
How can I draw a Lewis dot diagram for carbon dioxide ... How can I draw a Lewis dot diagram for carbon dioxide? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer Deevona Apr 18, 2015 1. 2. Two electrons (dots) make one bond (line). Answer link. Related questions. How is the Lewis structure of an ion written? ...
How To Draw Lewis Structure For Co2? | iLoveMyCarbonDioxide How To Draw Lewis Structure For Co2? Answer: Determine which atom is the center of the structure. A skeleton structure in which the other atoms are single-bonded to the central atom is drawn: Place an electron pair around each atom until it has an octet. The valence electrons in your trial structure (20) should be counted.
Lewis Structure Questions and Answers | Study.com Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a …
What is the Lewis structure for co2? - AskingLot.com In this manner, what is the electron dot structure for co2? Lewis dot structure is defined as the structure which denotes the valence electrons around the elements. The electrons are represented as dots in the structure. Carbon dioxide is the gas which is made by the combination of carbon and oxygen element.
Cl2 lewis structure, Molecular shape, Polar or Non-Polar ... Lewis dot structure for Cl2 (Chlorine gas) By looking at the above Cl2 lewis structure, we see both chlorine atoms completed their octet comfortably as both of them have 8 electrons around them. And no need to make any covalent bond in this lewis diagram because we got our stable lewis dot structure for Cl2.
Answered: Draw Lewis electron dot diagram of… | bartleby Draw Lewis electron dot diagram of compound CO2 to determine the molecular polarity by drawing the polarity vectors. Transcribed Image Text: Base your answer to the question on the information below and on your knowledge of chemistry. Wood is mainly cellulose, a polymer produced by plants. One use of wood is as a fuel in campfires, fireplaces ...
What is the dot diagram for carbon dioxide? - Answers A dot diagram (also called an Electron Dot Diagram, and a Lewis Structure) is a way to show the valence electrons that surround an element. See related link for a good lesson on how to make a dot ...
CO2 Lewis Structure | Lewis Dot Structure For Molecules ... Before we discuss the CO2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure. Lewis dot structure works on the octet rule, which means that all the atoms would have eight electrons in their valence shell except hydrogen.
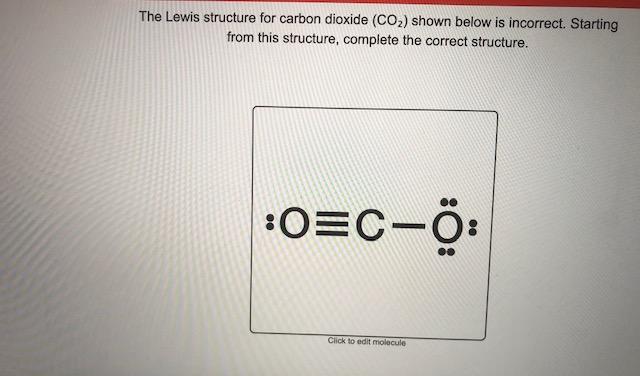
Draw the electron - dot structure of CO2 - Toppr Ask In an IIT(F) class a teacher asked the students to sketch the potential energy diagram for the formation of hydrogen molecule. Four students A, B, C and D drew the following diagrams a, b, c and d among which three were considered wrong and one correct by the teacher.
Carbon dioxide (CO2) lewis dot structure, molecular ... Valence electrons of CO2 show the outer shell electrons present around carbon and oxygen that can participate in the formation of the chemical bonds. Valence electrons of Carbon and Oxygen are represented as dots in the lewis diagram. To find the valence electron of carbon and oxygen we need the help of a periodic table.
CO2 Lewis Structure - Easy Hard Science CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.".
What is the Lewis dot diagram for carbon dioxide? - Quora Answer: There. That said, you could have found the exact same picture if you took 10 seconds to google "Lewis dot diagram, CO2. Sometimes I wonder.
CO2 Lewis Structure (2021 UPDATED) All You Need To Know 6 Steps on How to Draw CO2's Lewis Structure Calculate the total valence electrons found in a molecule. Carbon Valence Electron=4 Oxygen Valence electrons: 6*2 = 12 Total number of valence electrons = 16 Find the central atom, which is usually the one with the highest bonding sites, is the Carbon atom. Draw four dots around it.
What would be the electron dot structure of carbon dioxide ... Electron dot structures or Lewis dot formula - It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. Electron dot structures of carbon dioxide. The carbon is the central atom of this molecule. Oxygen atom contains 6 valence electrons which form 2 lone pairs.

















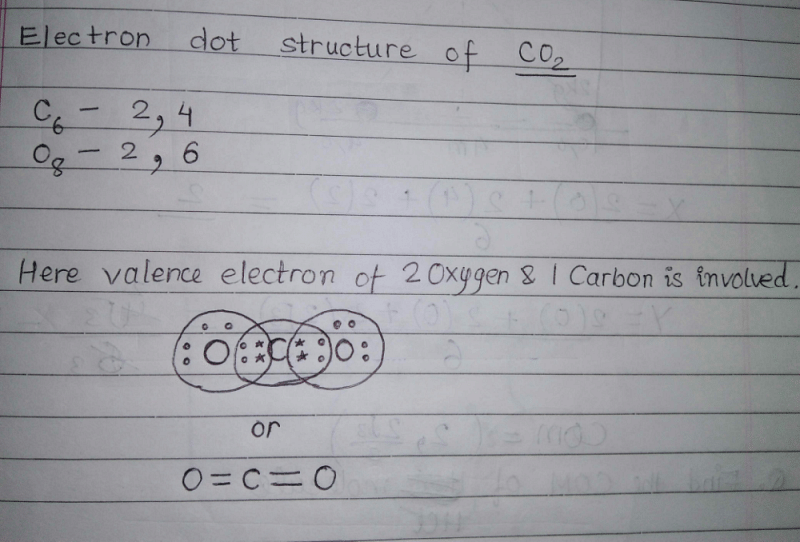





Comments
Post a Comment