42 energy level diagram for hydrogen
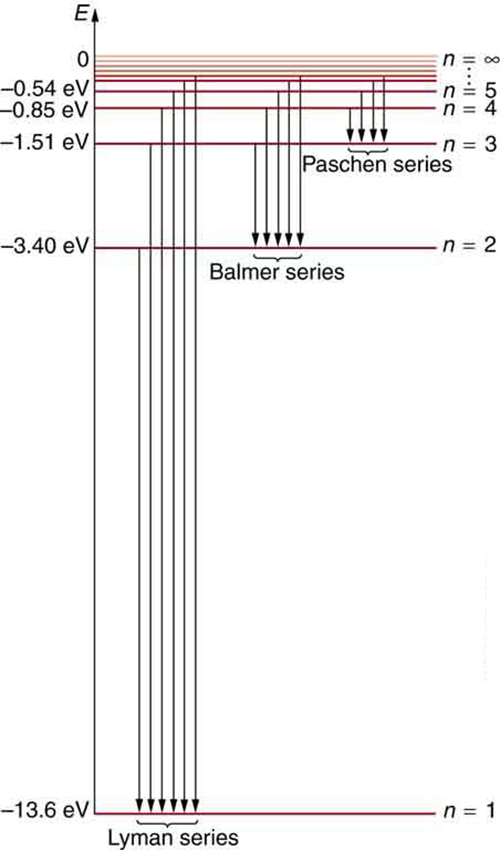
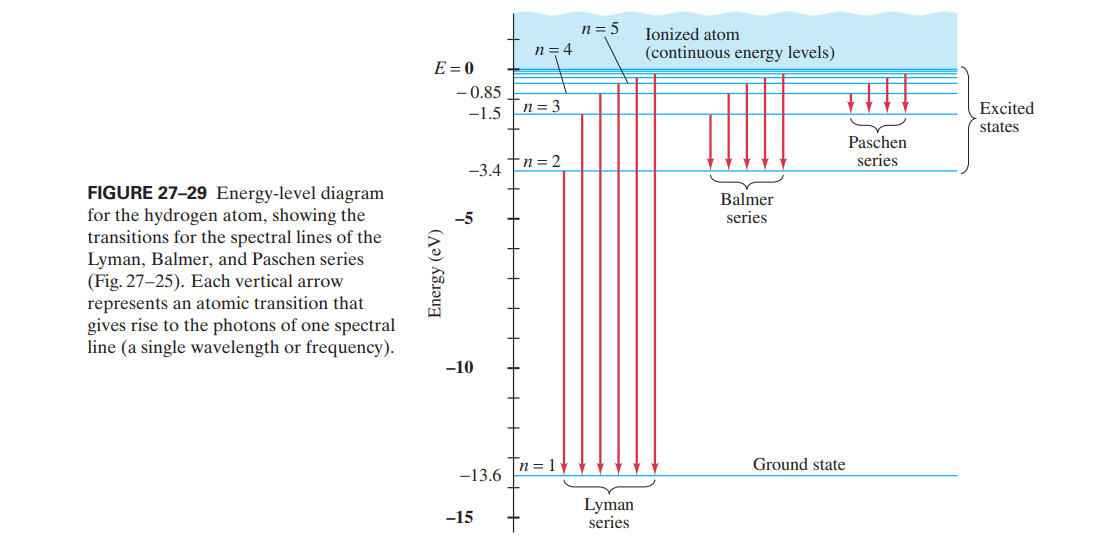
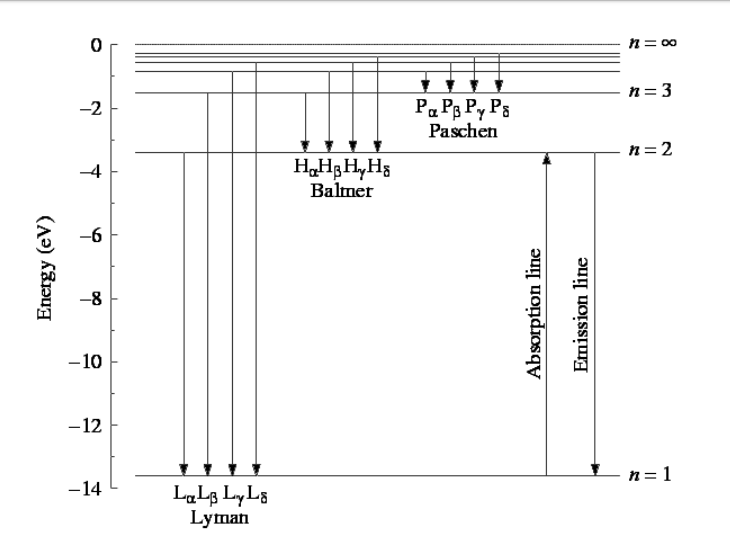
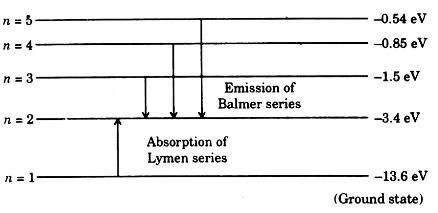
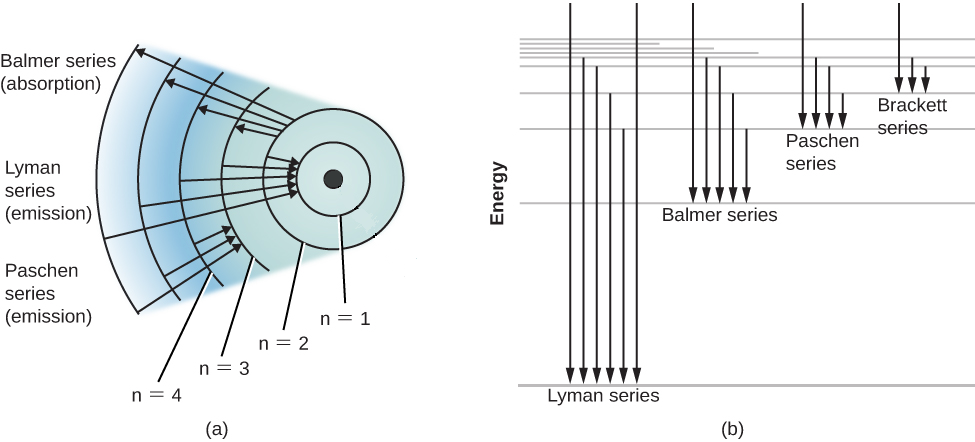
Energy-level diagram for hydrogen showing the Lyman, Balmer, and... Energy-level diagrams are used for many systems, including molecules and nuclei. A theory of the atom or any other system must predict its This figure shows an energy-level diagram for hydrogen that also illustrates how the various spectral series for hydrogen are related to transitions between... Relativistic Energy Levels for Hydrogen Atom A checkbox lets you view a schematic diagram of hydrogen energy levels for various levels of the theory. This includes also the hyperfine structure, from interaction between electron and nuclear magnetic moments. The transition in the level gives rise to the famous 1420 MHz (21 cm)...
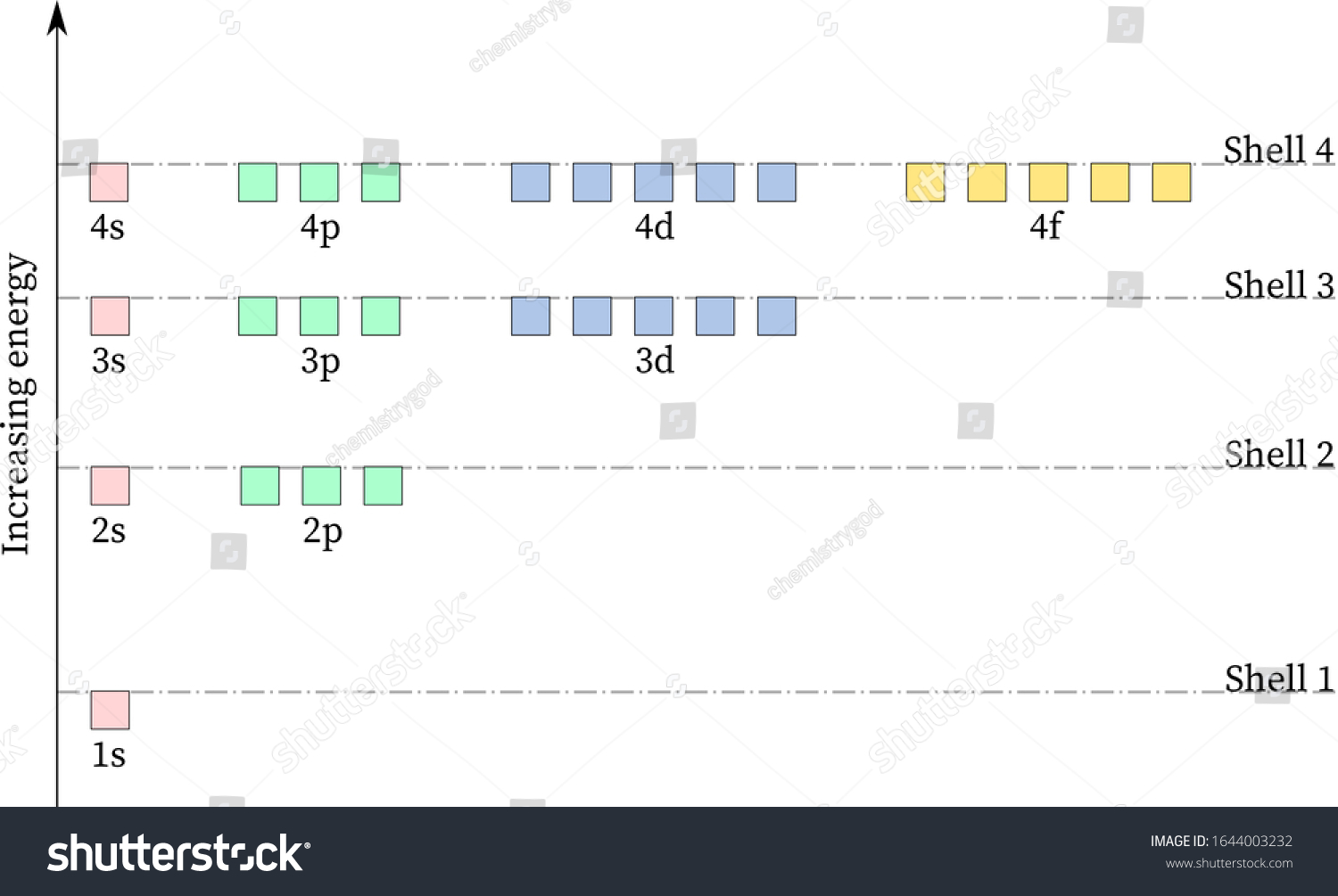
Energy of Orbitals - Calculating the Energy Level, Solved ... Calculating the Energy Level of an Orbital. In a single electron, Hydrogen-like atom, the orbital energy i.e. the energy of that one electron depends just on the principal quantum number (n). In orbitals chemistry when it comes to filling up the atom with electrons, the Aufbau principle tells the lower energy level orbitals always come first ...

Energy level diagram for hydrogen
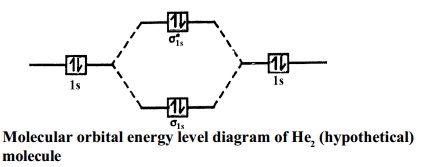
Energy level diagram for Molecular orbitals - Chemical ... 20.03.2019 · Energy level diagram for Molecular orbitals. The first ten molecular orbitals may be arranged in order of energy as follow: σ(1s) <σ ∗ (1s) < σ(2s) <σ ∗ (2s) < π(2p x) = π(2p y) < σ(2p z) < π ∗ (2p x) =π ∗ (2p y) <π ∗ ( 2p z) Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons ... PDF Chapter 18 - Electronic Structure of the Hydrogen Atom Energy of the Hydrogen-Like Atom. Given the constraint that 0∕2 = n we go back to. Energy levels of single electron bound to proton. orbitals. Energy of the Hydrogen-Like Atom. Remember Balmer series for hydrogen emission spectra obeys relation Energy Levels of Hydrogen Atom Note that the energy levels given in Equation (4.132) are independent of the quantum number , despite the fact that appears in the radial eigenfunction In addition to the quantized negative energy states of the hydrogen atom, which we have just found, there is also a continuum of unbound positive energy...
Energy level diagram for hydrogen. Partial Energy Level Diagram For Hydrogen - Wiring Diagram Source Light Energy Diagram Hydrogen Delapan Stanito Com. Synthesis Structure And Luminescence Of Er 3 Doped Y 3 Ga 5 O 12. Hydrogen Energy Diagram For Partial Free Wiring Diagram For You. Hydrogen Bonding Chemistry Libretexts. Energy Level Wikipedia. L23.1 Energy levels and diagram for hydrogen. - YouTube L23.1 Energy levels and diagram for hydrogen. Electricity - detailed contents In the energy level diagram(below), energies have to be measured relative to one another. Remember the ground state is the lowest level and ionisation is the highest. So you would expect the ground state be zero. Energy level and Bohr model of hydrogen | 3 Important Points Energy level for Hydrogen image credit :Ivan Ramirez, Energy-Level Diagrams for Hydrogen Energy level means of evaluating the amount of energy electron may require and will be released during The emission spectrum of atomic hydrogen was divided into numerous spectral series and...
Vehicle Technologies Office | Department of Energy Last year, vehicles transported 11 billion tons of freight, more than $32 billion worth of goods each day, and moved people more than 3 trillion vehicle-miles. The U.S. Department of Energy's Vehicle Technologies Office provides low cost, secure, and clean energy technologies to move people and goods across America. New Technology Lowers Cost of Green Hydrogen Energy … 31.01.2022 · Schematic diagram of the AEMWE, where the catalyst layer consists of ionomers and catalysts. Image Credit: Korea Institute of Science and Technology (KIST). Specifically, there has been an active study and development of water electrolysis technology that removes hydrogen from water to generate green energy and shuns the emission of greenhouse gases. … PDF Quantum Physics III Chapter 2: Hydrogen Fine Structure The energy levels are enumerated using a principal quantum number n, an integer that must be greater or equal to one Using this notation for coupled basis multiplets the diagram of hydrogen atom energy eigenstates becomes: 30 chapter 2. hydrogen atom fine structure. PDF A. Calculations of the energy levels of the hydrogen atom Energy-level Diagram for Hydrogen. Estimate the values of the wavelengths of the lines you observe! Do this especially for hydrogen, using the measuring device in the spectroscope.
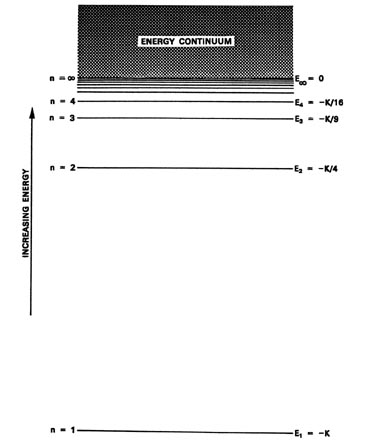
Negative energy levels in the diagram for a hydrogen atom The higher the number of the shell (n), the higher is the energy level of the electron. However, why was it necessary to have negative values. So for example, when $n=1$, the energy could be $5 eV$ and for $n=2$, $6 eV$... having positive values could also have supported the idea that as $n$ increases... atomic hydrogen emission spectrum The next few diagrams are in two parts - with the energy levels at the top and the spectrum at the bottom. If an electron fell from the 6-level, the fall is The infinity level represents the highest possible energy an electron can have as a part of a hydrogen atom. So what happens if the electron exceeds... Proton Exchange Membrane Water Electrolysis as a Promising ... 15.02.2018 · Energy capture as hydrogen via water electrolysis has been gaining tremendous interest in Europe and other parts of the world because of the higher renewable penetration on their energy grid. Hydrogen is an appealing storage medium for excess renewable energy because once stored, it can be used in a variety of applications including power generation in … PDF Lecture 2 Hyperfine structure. Energy levels of the hydrogen atom so far Prediction of the relativistic (Dirac equation) theory for hydrogen energy levels . Feynman diagrams for Lamb shift: Element #1: Double line represents bound electrons Element #2: Wavy line represents a virtual photon.
The energy level diagram for the reaction of hydrogen with chlorine... An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction as the reaction proceeds ( called 'extent of the reaction' below). The transition state is a stage during the reaction at which chemical bonds are partially broken and formed.
Hydrogen energies and spectrum | Hydrogen Energy Levels The basic hydrogen energy level structure is in agreement with the Bohr model. Common pictures are those of a shell structure with each main shell associated with a value of the principal quantum number n. This Bohr model picture of the orbits has some usefulness for visualization so long as it is realized...
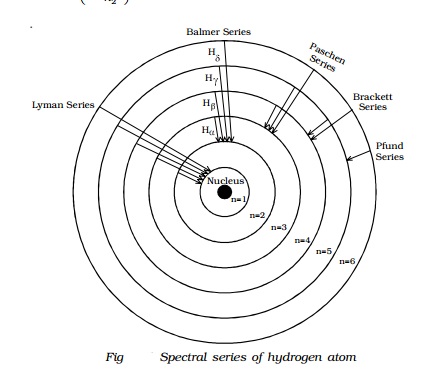
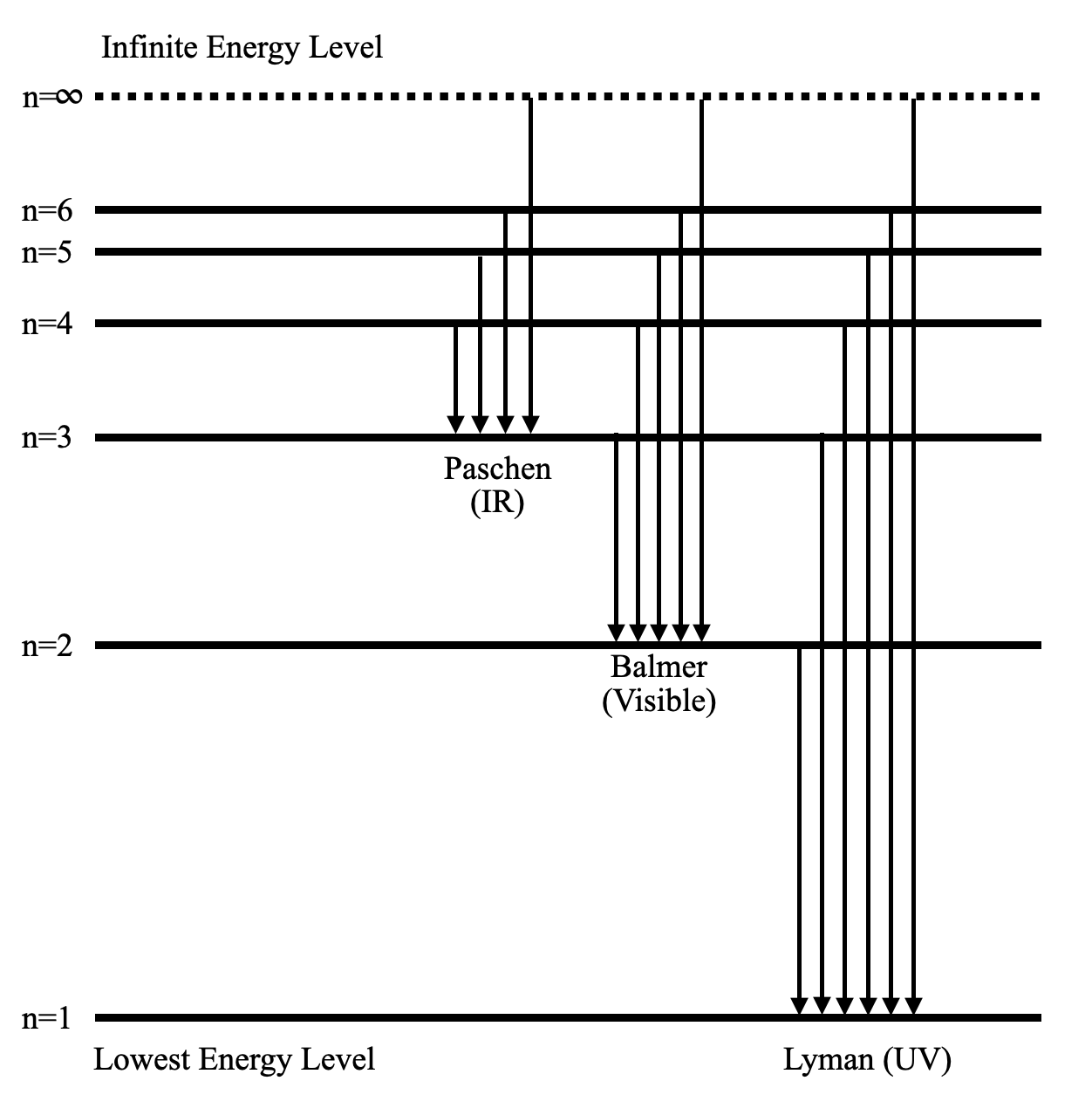
Hydrogen spectral series - Wikipedia (Redirected from Hydrogen frequencies). The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom.
Hydrogen energy-level diagram - Big Chemical Encyclopedia Energy level diagram for hydrogen molecule, H2, and separated atoms H R = 00) and He R = 0). R = the Rydberg constant = 13.6057 eV = 0.5 a.u. (atomic unit of energy). Value from ionization potential of He (Is 2p P). Value from ionization potential of H2. The experimental ionization potentials are quite...
Figure 7. Energy-level diagram for hydrogen showing the Lyman... Energy-level diagrams are used for many systems, including molecules and nuclei. A theory of the atom or any other system must predict its Figure 7 shows an energy-level diagram for hydrogen that also illustrates how the various spectral series for hydrogen are related to transitions between...
Why do energy level diagrams differ for hydrogen and... - Quora You can actually use these energy level diagrams for helium, which (normally) has 2 electrons. What matters is that hydrogen and helium only have one orbital, which can very easily be described. The orbital has no complex functions regarding probability density, which makes the equation different.
Energy Level Diagram For Hydrogen - Mini Physics - Learn Physics Home A Level Quantum Physics & Lasers (A Level) Energy Level Diagram For Hydrogen. The highest energy level n = ∞ corresponds to an energy state whereby the electron is no longer bound to the atom. (the electron has escaped from the atom.)
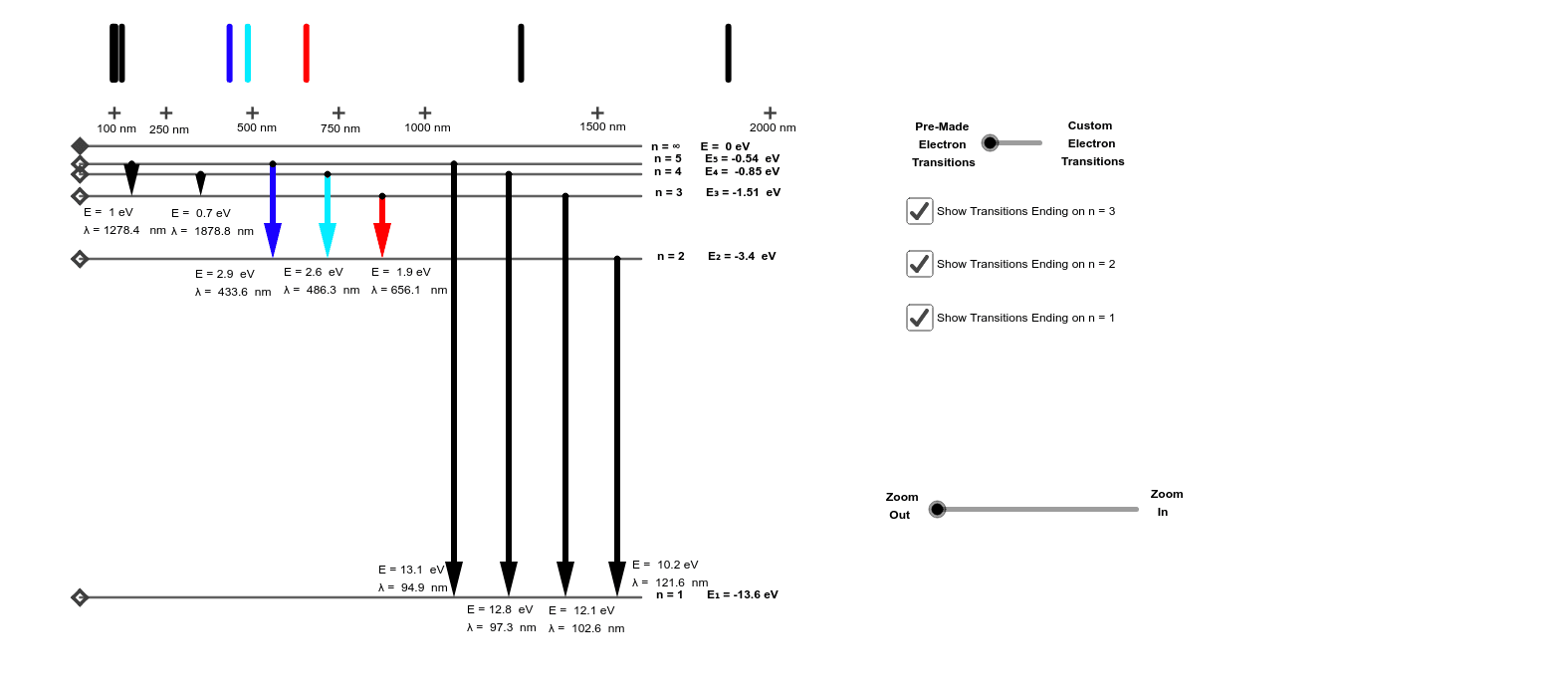
Energy Level Diagrams - Hydrogen - GeoGebra © 2021 GeoGebra. Energy Level Diagrams - Hydrogen. Author: Barb Newitt. Topic: Diagrams.
Lesson Explainer: Electron Energy Levels | Nagwa The diagram shows a hydrogen atom. The electron shown transitions between two energy levels of the atom. Which energy level is the electron initially in? Which energy level does the electron transition to? Answer . Part 1. The energy levels are counted from the inside out. We see that the electron is initially in the outermost level, the fourth ring from the center. The electron …
Energy level diagrams and the hydrogen atom Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is …
Nuclear fusion in the Sun - Energy Education The energy from the Sun - both heat and light energy - originates from a nuclear fusion process that is occurring inside the core of the Sun.The specific type of fusion that occurs inside of the Sun is known as proton-proton fusion.. Inside the Sun, this process begins with protons (which is simply a lone hydrogen nucleus) and through a series of steps, these protons fuse together …
Bohr's model of hydrogen (article) | Khan Academy Atomic Energy Levels. Bohr model radii (derivation using physics). Bohr model energy levels. Absorption and emission. Emission spectrum of hydrogen. Bohr's model of hydrogen. This is the currently selected item.
Hydrogen Energy Level Diagram Economic Economy. Details: Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. › Get more: Partial energy diagram for hydrogenView Economy. Hydrogen Energy Levels - Georgia State University.
Energy Levels of Neutral Hydrogen ( H I ) Energy Levels of Neutral Hydrogen ( H I ). Configuration. Term. J. Level(cm-1). Ref. 1s.
Hydrogen Energy Levels Calculator The Hydrogen energy levels calculator helps you to compute the energy levels of the hydrogen atom and hydrogen-like atoms and ions. You will also find answers to questions like: how many energy levels does hydrogen have or how to calculate ionization energy of hydrogen.
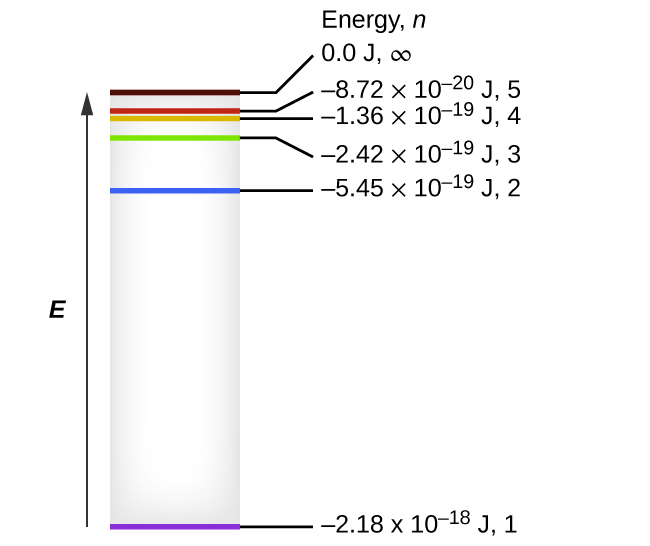
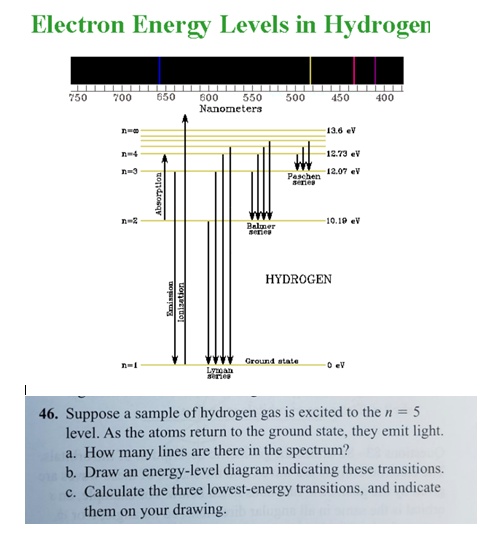
Energy Levels in Atoms - Sloan Digital Sky Survey If an electron is in the first energy level, it must have exactly -13.6 eV of energy. If it is in the second energy level, it must have -3.4 eV of energy. An electron in a hydrogen atom cannot have -9 eV, -8 eV or any other value in between. Let's say the electron wants to jump from the first energy level, n = 1, to the second energy level n ...
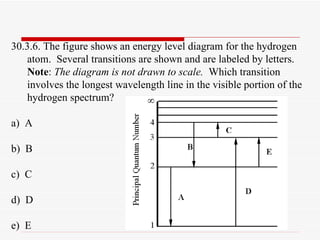
Energy Level and Transition of Electrons | Brilliant Math & Science Wiki The energy level of the electron of a hydrogen atom is given by the following formula, where. nn. n denotes the principal quantum number The figure below shows the electron energy level diagram of a hydrogen atom. Observe how the lines become closer as.
Energy Level Diagram For Hydrogen Atoms The Energy level diagram for the Hydrogen Atom indicates the different series of lines that is observed in the Spectrum. When the Hydrogen Atom absorbs a photon, it results in causing the Electron to experience a transition to the higher Energy level, where n = 1, n = 2 as an example.
Energy Flow in an Ecosystem (With Diagram) The radiant energy is in the form of electromagnetic waves which are released from the sun during the transmutation of hydrogen to helium. The chemical energy stored in the food of living organisms is converted into potential energy by the arrangement of the constituent atoms of food in a particular manner. In any ecosystem there should be unidirectional flow of energy. This …
Hydrogen Energy Levels Flashcards | Quizlet The upper right panel panel "Energy Level Diagram" shows the energy levels vertically with correct relative spacing between the levels. The "Photon Selection" panel bottom left allows you to pick the energy/wavelength/frequency (all separately shown) of the photon to shoot at the hydrogen atom.
Bohr model - Wikipedia Models depicting electron energy levels in hydrogen, helium, lithium, and neon. The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the hydrogen atom...
Energy Levels of Hydrogen Atom Note that the energy levels given in Equation (4.132) are independent of the quantum number , despite the fact that appears in the radial eigenfunction In addition to the quantized negative energy states of the hydrogen atom, which we have just found, there is also a continuum of unbound positive energy...
PDF Chapter 18 - Electronic Structure of the Hydrogen Atom Energy of the Hydrogen-Like Atom. Given the constraint that 0∕2 = n we go back to. Energy levels of single electron bound to proton. orbitals. Energy of the Hydrogen-Like Atom. Remember Balmer series for hydrogen emission spectra obeys relation
Energy level diagram for Molecular orbitals - Chemical ... 20.03.2019 · Energy level diagram for Molecular orbitals. The first ten molecular orbitals may be arranged in order of energy as follow: σ(1s) <σ ∗ (1s) < σ(2s) <σ ∗ (2s) < π(2p x) = π(2p y) < σ(2p z) < π ∗ (2p x) =π ∗ (2p y) <π ∗ ( 2p z) Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons ...



















Comments
Post a Comment