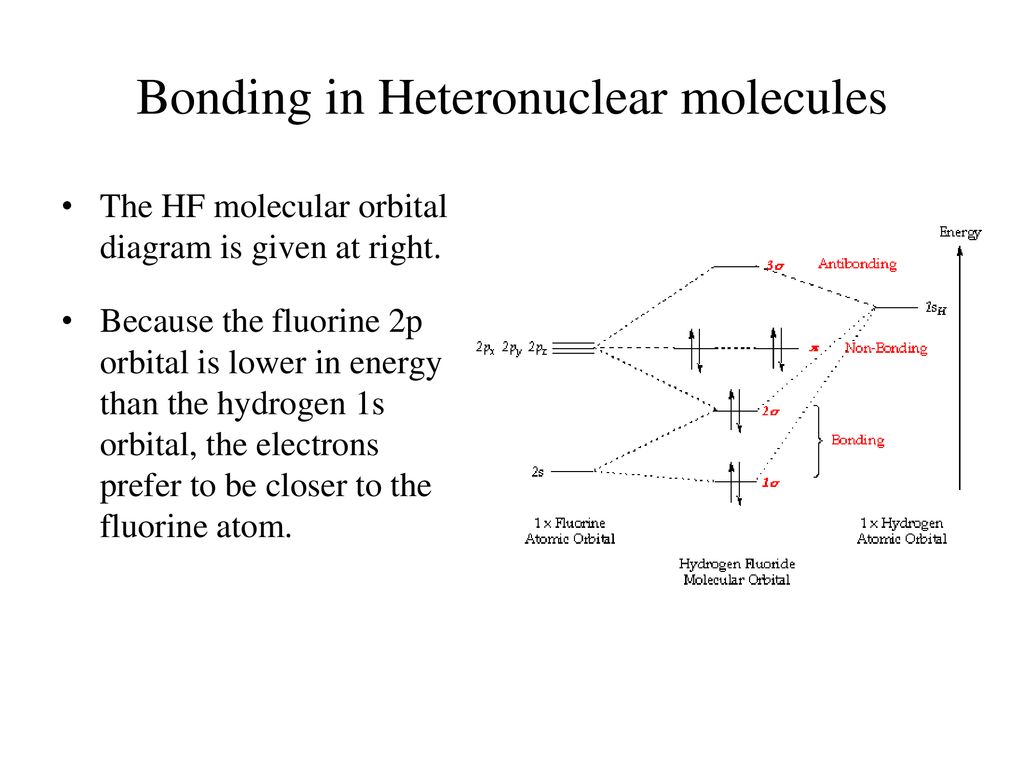
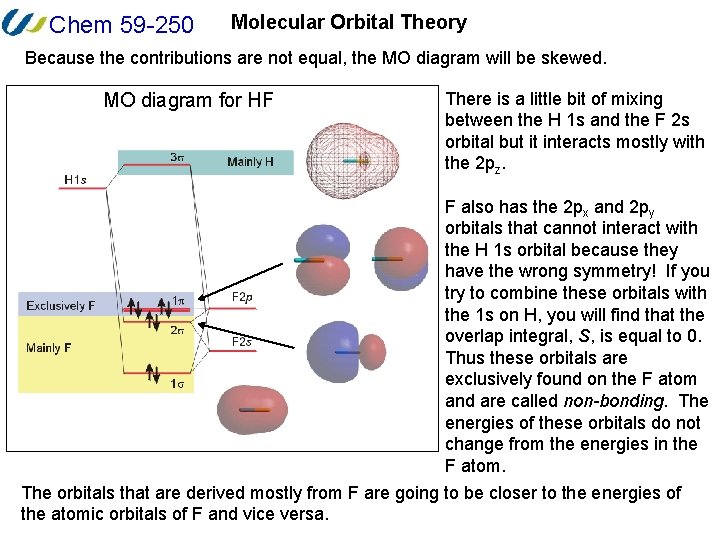
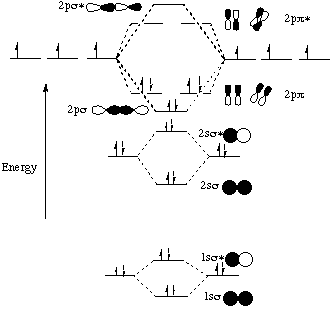
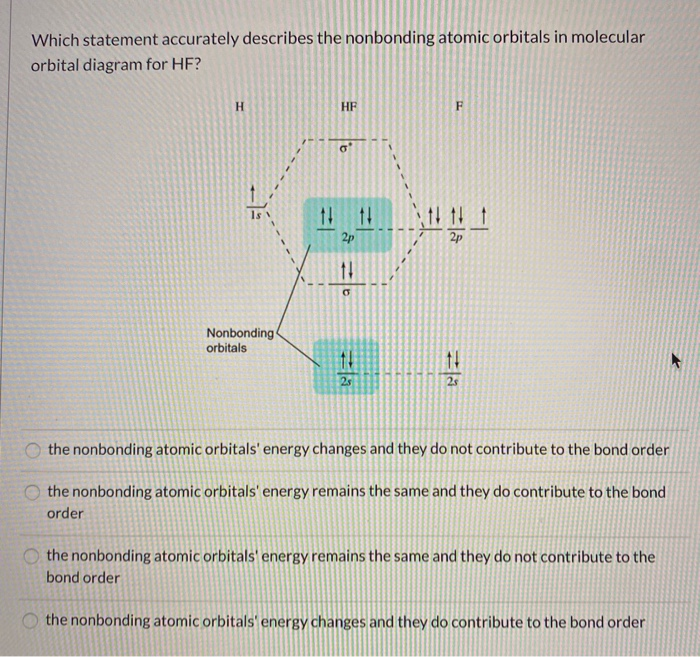
39 molecular orbital diagram of hf
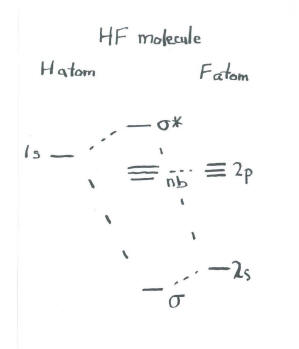
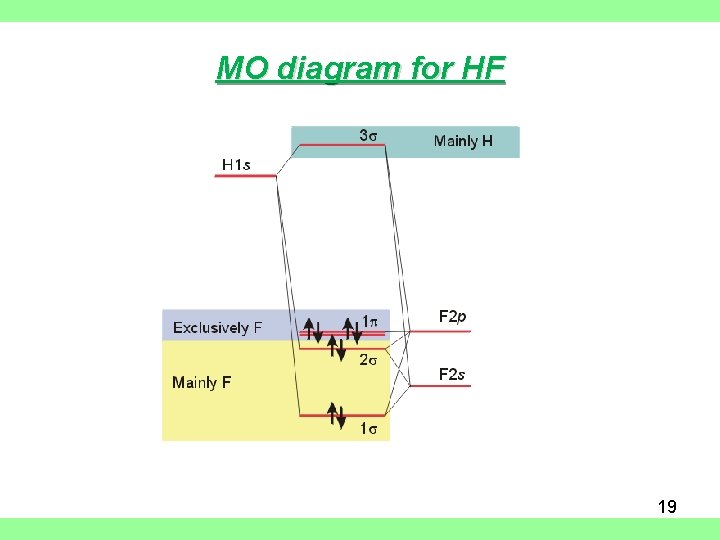
Drawn below is an incomplete molecular orbital (MO) diagram for the molecule HF. ... A nonbonding electron is promoted to an anti bonding orbital.3 pages HF — Hydrogen fluoride is another example of a heteronuclear molecule. It is slightly different in that the π orbital is non-bonding, as well as the 2s σ.
In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already ...3 answers · 33 votes: The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation ...

Molecular orbital diagram of hf
The qualitative molecular orbital diagram, as depicted in Fig. 2, also shows the two-center three-electron (2c-3e) σ half-bonding character of HF − (X 2 Σ + ) ... Molecular Orbital Diagram for the HF Molecule — Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the ... There are two molecular orbitals for hydrogen, the lower energy orbital has its greater electron density between the two nuclei. This is the bonding molecular ...
Molecular orbital diagram of hf. In hydrogen fluoride (HF), the hydrogen 1s orbital can mix with the fluorine 2pz orbital to form a sigma bond because experimentally, the energy of 1s of ... 1 answerIt is a diatomic molecule that contains two different atoms in which one is more electronegative. And the one which is more electronegative will have lower ... There are two molecular orbitals for hydrogen, the lower energy orbital has its greater electron density between the two nuclei. This is the bonding molecular ... Molecular Orbital Diagram for the HF Molecule — Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the ...
The qualitative molecular orbital diagram, as depicted in Fig. 2, also shows the two-center three-electron (2c-3e) σ half-bonding character of HF − (X 2 Σ + ) ...





















Comments
Post a Comment