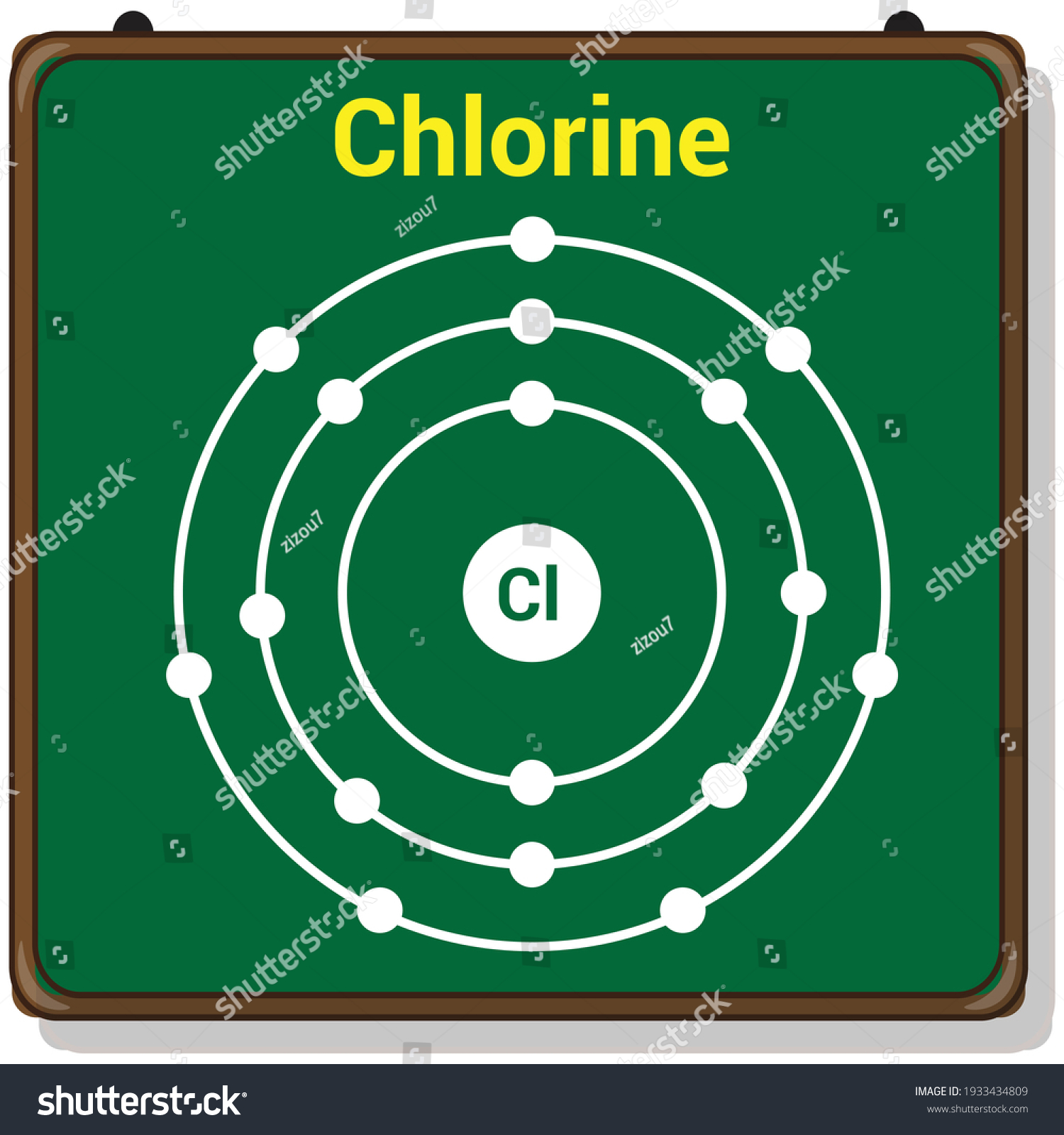
38 bohr diagram for chloride
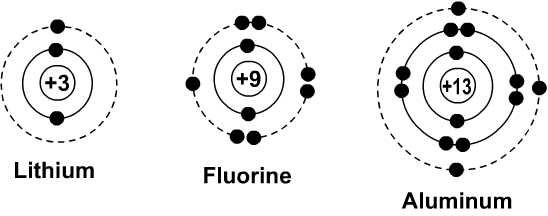
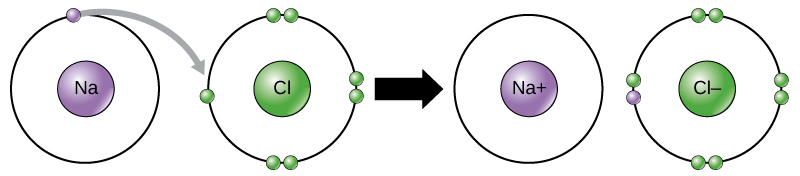
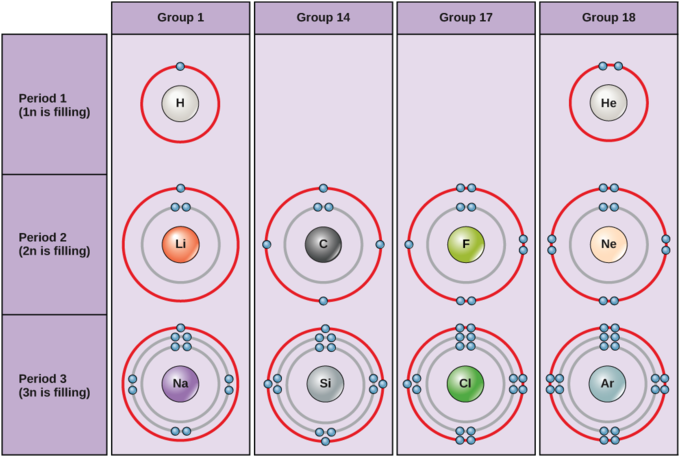
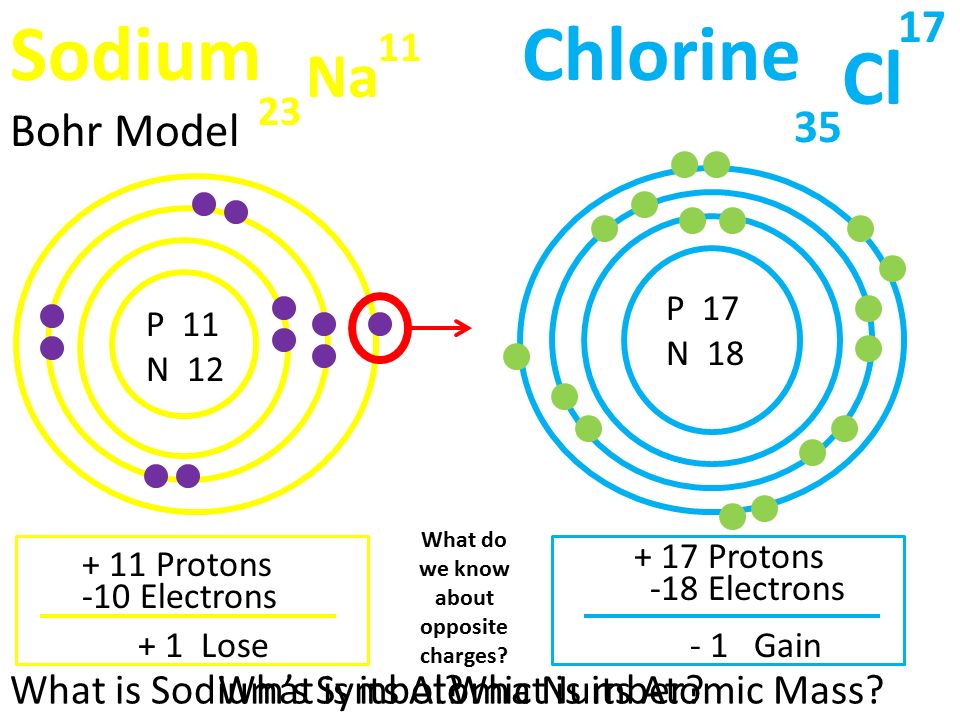
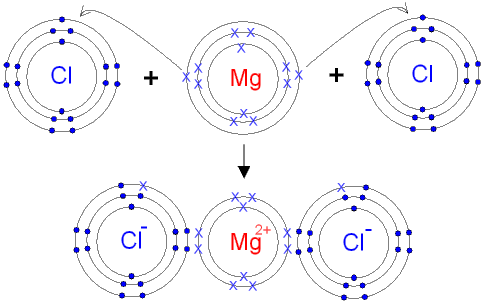
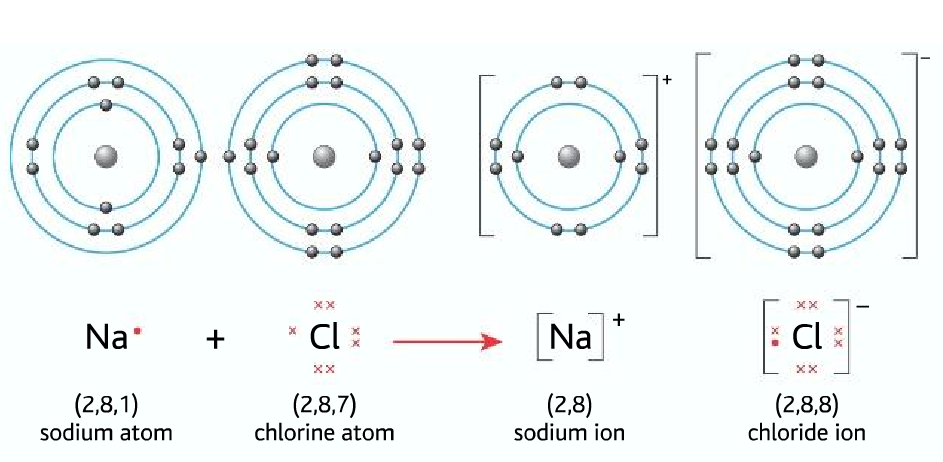
Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. You can use Bohr diagrams to show ionic bonding for simple compounds. The formation of the ionic compound sodium chloride is shown in the figure below. An ionic compound forms when an electron from a metal atom transfers to a non-metal atom, creating oppositely charged ions. Sodium chloride (NaCl) has a ratio of ions of 1:1.
NH 4 Cl adopts the caesium chloride structure. The NH 4+ cation occupies the cubic site at the centre of the cell and is able to hydrogen bond with the Cl - ions at the corners of the cell.
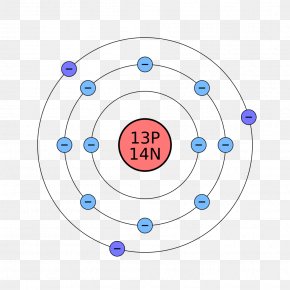
Bohr diagram for chloride
A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing. To draw the Bohr diagram for "NaCl", we should first draw the individual diagrams for both "Na" and "Cl". ... This chapter is most relevant to Section F8(ii) from the 2017 CICM Primary Syllabus, which expects the exam candidates to be able to "describe the carbon dioxide carriage in blood including the Haldane effect and the chloride shift". Presumably, the Bohr effect was left out because it is an implied element, because surely you could not have a discussion of the Haldane effect without it. The heat of formation of sodium chloride (ΔH f 0) from the sodium metal and chlorine gas can be experimentally measured. Na (s) + 1 2 \frac{1}{2} 2 1 Cl 2 (g) → NaCl(s) ΔH f 0 = -411kJ/mol. The formation of ionic solid sodium chloride form solid sodium metal and gaseous chlorine is not a single step process but goes through several processes.
Bohr diagram for chloride. Facts Date of Discovery: 1774 Discoverer: Carl Wilhelm Scheele Name Origin: From the Greek word khlôros (green) Uses: Water purification, bleaches Obtained From: Salt Related Links Note: The external links below are not a part of this site and their content is not the responsibility of this site Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ... A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing. To draw the Bohr diagram for "NaCl", we should first draw the individual diagrams for both "Na" and "Cl". The atomic number of "Na" is 11, so it has 11 electrons. The Bohr model of the atom. ... If you look at the diagrams of the sodium and chlorine atoms you can see that sodium normally has eleven electrons in shells around the nucleus. You can also see that chlorine has seventeen electrons around its nucleus. ... now contains only ten electrons and the new chloride ion (an anion) has eighteen electrons.
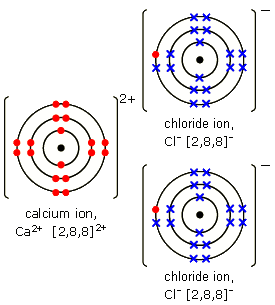
Bohr Diagram For Calcium. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Calcium 2,8,8,2. Ca. Calcium atomic orbital and chemical bonding information. You may have an easy way to know the number of electrons in a neutral atom, but the placement of . B. Compare a Bohr diagram and a Lewis diagram. Explain how they are: similar. different. Draw the Bohr Model diagram for each of the following atoms. Neon Atom Fluorine atom Fluorine Ion Sodium Atom Sodium Ion Draw the Bohr model diagram for each of the following compounds. Carbon Dioxide (CO2) Ammonia (NH3) Calcium Chloride (CaCl2) Bohr Diagram For Calcium Chloride Wiring Diagram. In A Calcium Atom The Bohr Model Of The Atom Is 2 8 8 2. Bohr Diagram For Calcium Atom Wiring Diagrams Folder. 4 1 Atomic Models Of The Twentieth Century Chemistry. What Is The Atomic Structure Of Calcium According To Bohr S. Electronic Structure And Chemical Bonding. 2.3 Quantum Numbers. Get Started. Active 1 year, 2 months ago. Select Page. Average Atomic Mass: 40.08 4. 1. The Bohr diagram for boron shows a central nucleus containing five protons. When electrons return to … bohr diagram for calcium. Ca 41. Calcium chloride is also known as CaCl2 in scientific ...
The solid and the solution is separated and the supernatant solution is recycled for further evaporation. Lithium chloride is a solid which absorbs water to form a hydrate, LiCl.H 2 O. LiCl. Lithium Chloride. Density. 2.07 g/cm³. Molecular Weight/ Molar Mass. 42.394 g/mol. The molecules or ions surrounding the central metal ion are called ligands. Simple ligands include water, ammonia and chloride ions. Figure 24.1. 1: Simple Bohr diagram bonding in water, ammonia, and the chloride ion. What all these have got in common is active lone pairs of electrons in the outer energy level. Bohr-Rutherford diagram of sodium chloride? Bohr-Rutherford diagram of The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2. Both elements have three electron shells. Sodium has one electron in its outer shell and chlorine has seven. Neither of them has an outer shell that is filled. NaCl, sodium chloride, is an IONIC compound, which means electrons are TRANSFERRED from one atom to another.You'll have to draw the Bohr-Rutherford Diagram o...

Beryllium Chloride Bohr Model Atom Crystal Structure Png 1100x760px Beryllium Chloride Atom Atomic Mass Atomic Number Ballandstick Model Download Free
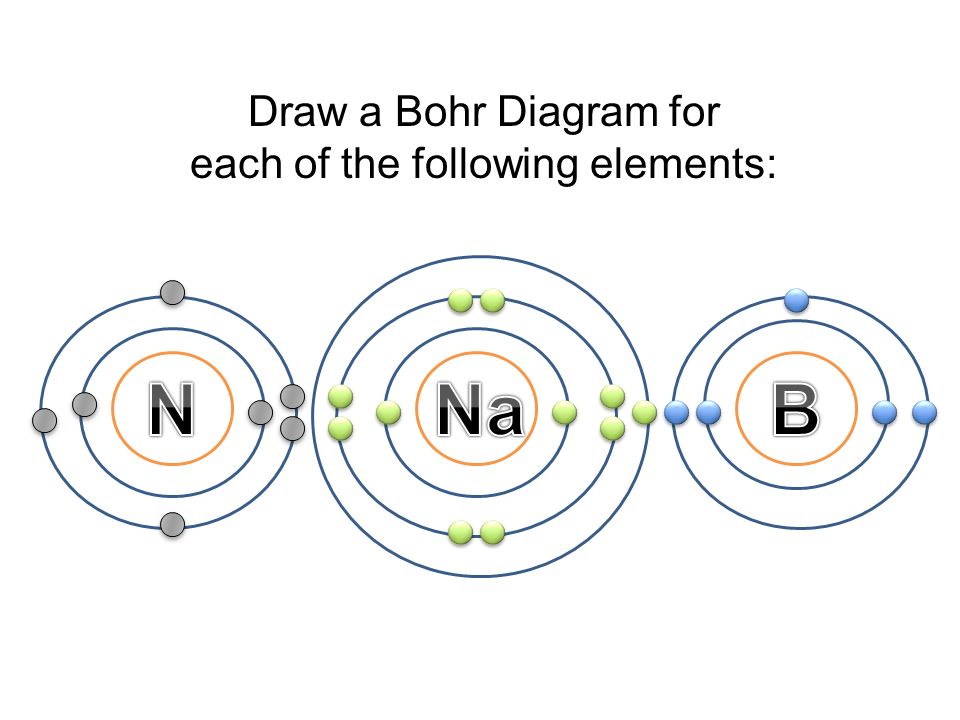
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
2021-11-27. Create. 2005-03-26. Lithium chloride appears as colorless crystals or powder. Low toxicity. CAMEO Chemicals. Lithium chloride is a metal chloride salt with a Li (+) counterion. It has a role as an antimanic drug and a geroprotector. It is an inorganic chloride and a lithium salt.
Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds 2 electrons. The second holds 8. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. The remaining 2 are placed in the third electron shell, which is full when it holds 8 electrons.
d) Magnesium Chloride, MgCl 2 Part 2 - Bohr Diagrams of Covalent Compounds 1. Start by drawing the Bohr diagram of each atom in the compound. Examples: Sulfur, S Chlorine, Cl 2. To show a covalent bond, allow the valence shell of each atom to overlap, such that each shell seems to have a full complement of electrons. Example:
The Lewis Electron-Dot Symbols of Elements. Gilbert N Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen the Bohr's diagram for the first 18 elements. Sometimes it is more convenient to represent the elements by its Lewis electron dot symbol.
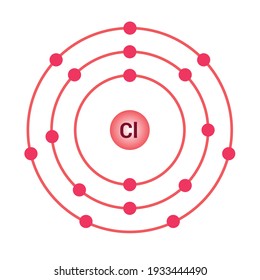
The Bohr Model of Chlorine(Cl) has a nucleus that contains 18 neutrons and 17 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Chlorine contains 7 electrons that also called valence electrons.
The ionic compounds lithium fluoride and beryllium chloride. Bohr Diagrams Bohr diagrams for atoms imagine a nucleus surrounded by electron shells corresponding to principle quantum numbers for ...
Chlorine has 2 electrons in its first shell, 8 in its second and 7 in its third.Check me out: http://www.chemistnate.com
Bohr Diagrams of Ions Draw Bohr Diagrams of the following ions. Make sure you include: The ionic charge Electrons in the energy shells The Chemical Symbol Lithium Magnesium Oxygen Chlorine Draw Bohr diagrams for the following ionic compounds LiCl MgO MgCl 2 H 2 S .
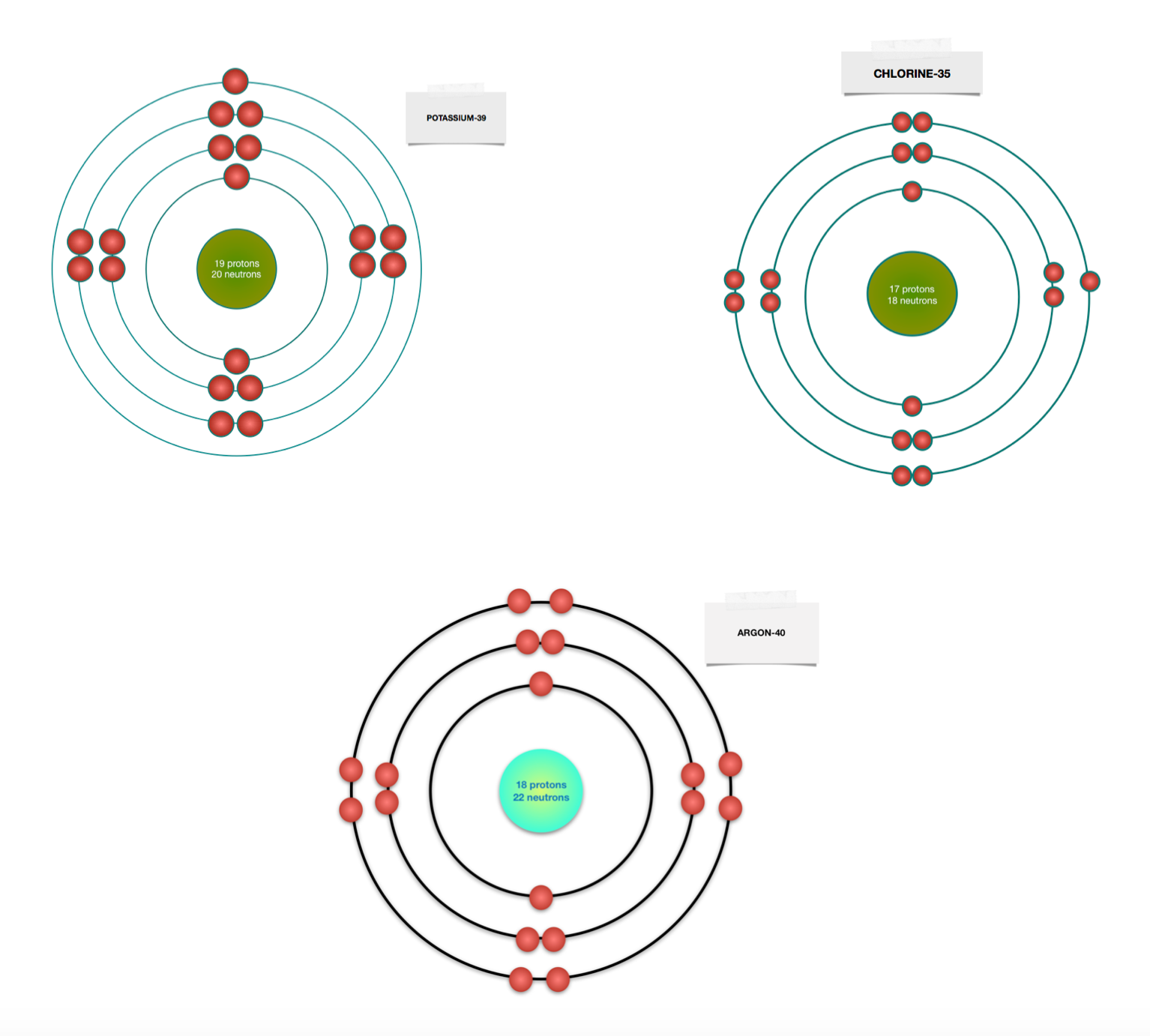
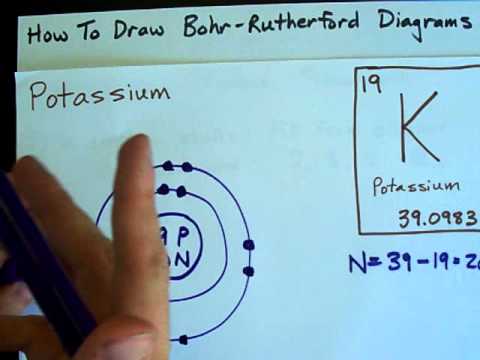
The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the ...
Diagram Bohr Diagram For Calcium Chloride Full Version Hd Review Of Bohr Models Answer Key Share this post. 0 Response to "32 Bohr Diagram Of Calcium" Post a Comment. Newer Post Older Post Home. Subscribe to: Post Comments (Atom) Iklan Atas Artikel. Iklan Tengah Artikel 1.
The heat of formation of sodium chloride (ΔH f 0) from the sodium metal and chlorine gas can be experimentally measured. Na (s) + 1 2 \frac{1}{2} 2 1 Cl 2 (g) → NaCl(s) ΔH f 0 = -411kJ/mol. The formation of ionic solid sodium chloride form solid sodium metal and gaseous chlorine is not a single step process but goes through several processes.
This chapter is most relevant to Section F8(ii) from the 2017 CICM Primary Syllabus, which expects the exam candidates to be able to "describe the carbon dioxide carriage in blood including the Haldane effect and the chloride shift". Presumably, the Bohr effect was left out because it is an implied element, because surely you could not have a discussion of the Haldane effect without it.

Bohr Model Atom Electron Configuration Argon Calcium Molecular Atom Chemical Element Symmetry Electron Png Pngwing
A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing. To draw the Bohr diagram for "NaCl", we should first draw the individual diagrams for both "Na" and "Cl". ...

Notes The Bohr Model Happy Atoms Ions What Atom Element Do You Think This Is How Can You Tell Ppt Download
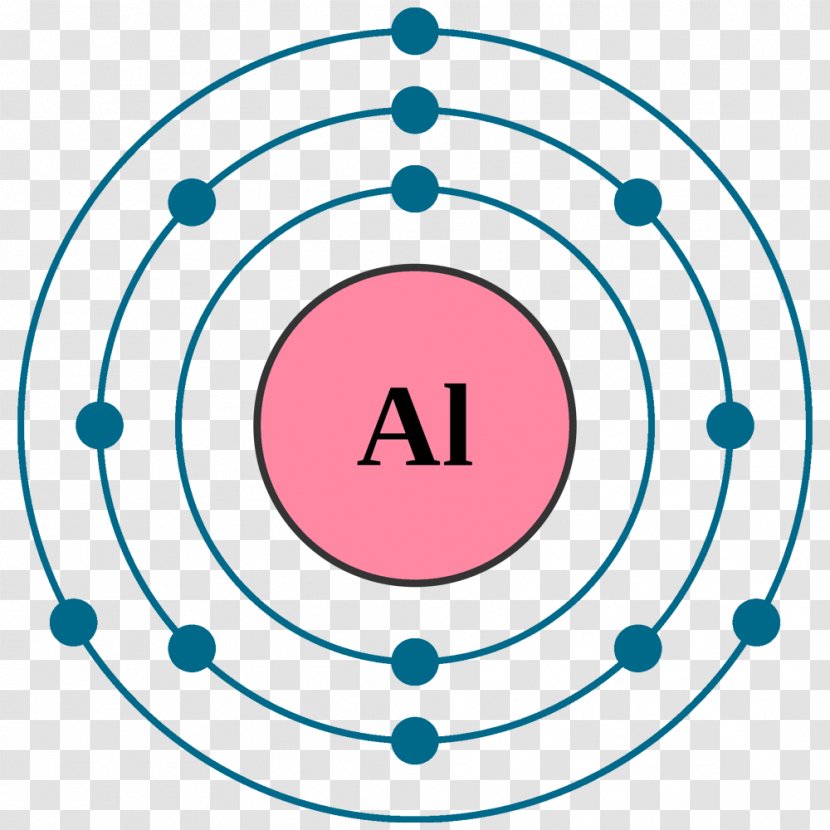
Atom Bohr Model Electron Configuration Chlorine Lewis Structure Aluminum Chemical Eleme Transparent Png











Comments
Post a Comment