42 phase diagram for chocolate
In its solid phase, the crystal bonds in tempered chocolate are stable, meaning greater force will be necessary to change or distort its shape. A properly tempered chocolate remains stable at room temperatures, about 68-77ºF (20-25ºC). When heat is applied, usually at 96ºF—the melting point for chocolate, which is a couple of degrees lower ... The production of chocolate Introduction. Chocolate is a key ingredient in many foods such as milk shakes, candy bars, cookies and cereals.It is ranked as one of the most favourite flavours in North America and Europe (Swift, 1998). Despite its popularity, most people do not know the unique origins of this popular treat.
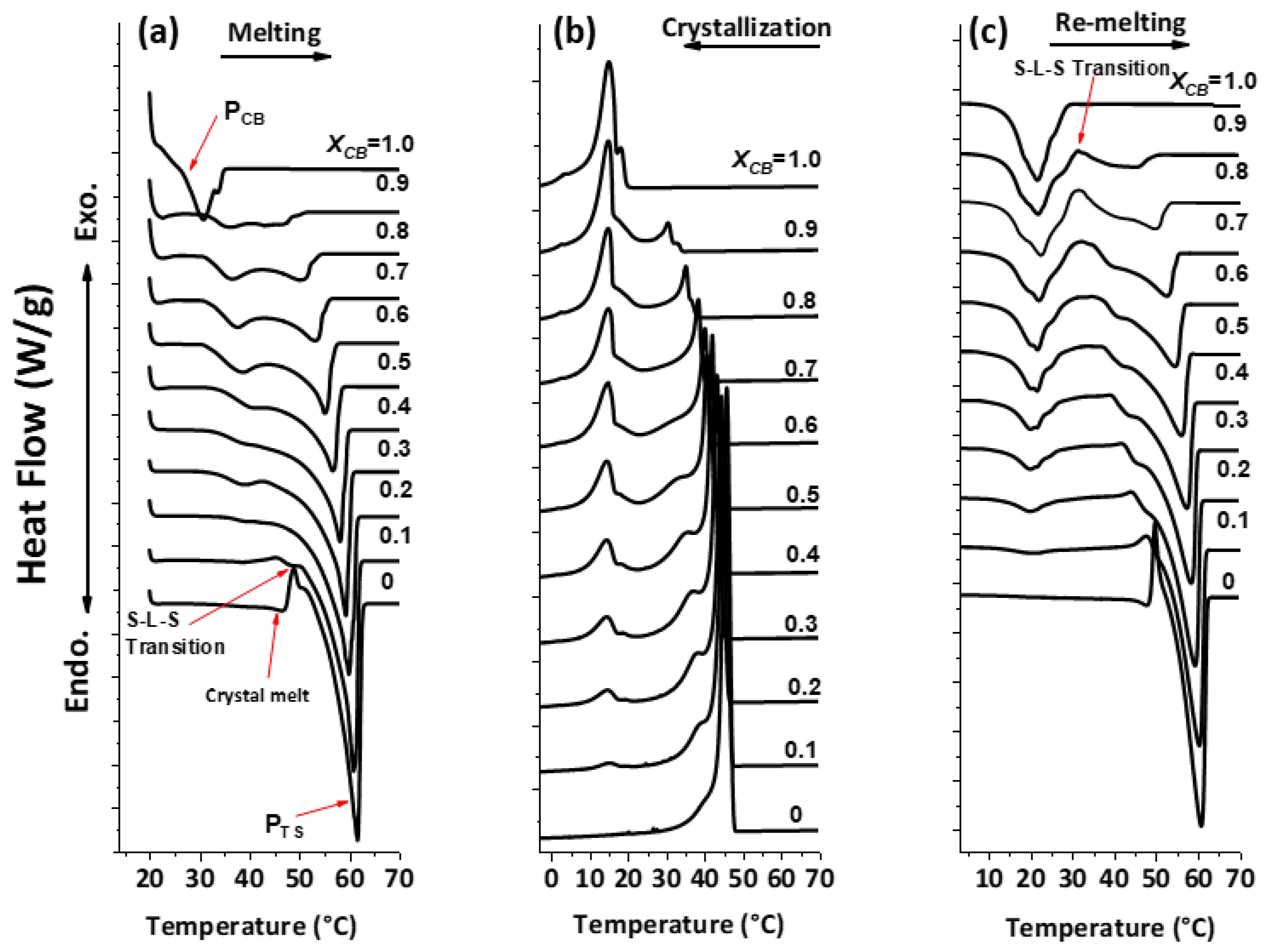
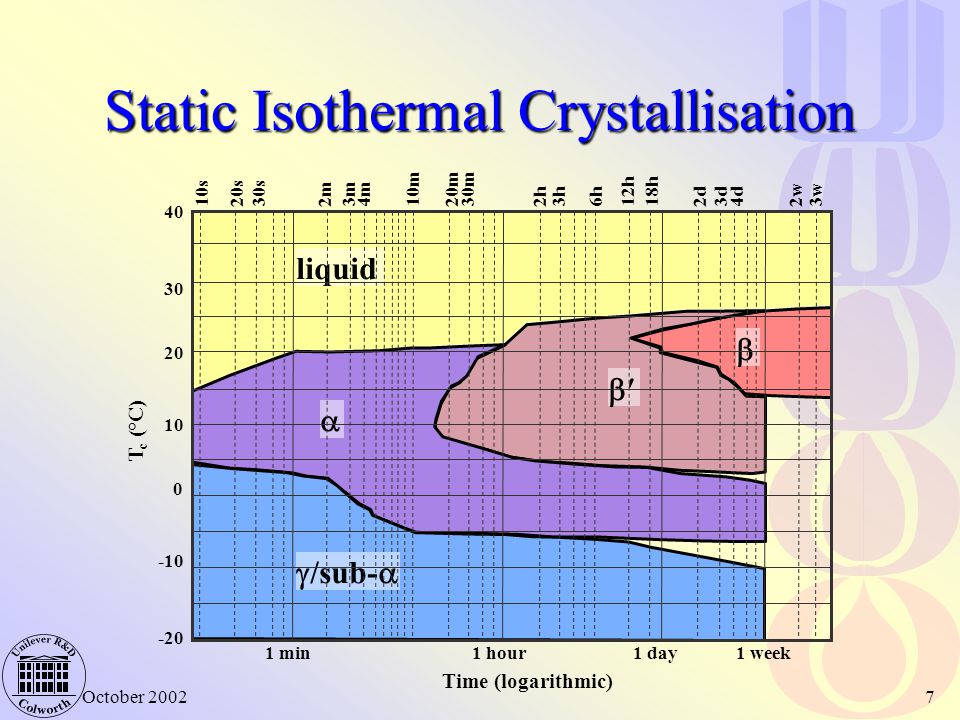
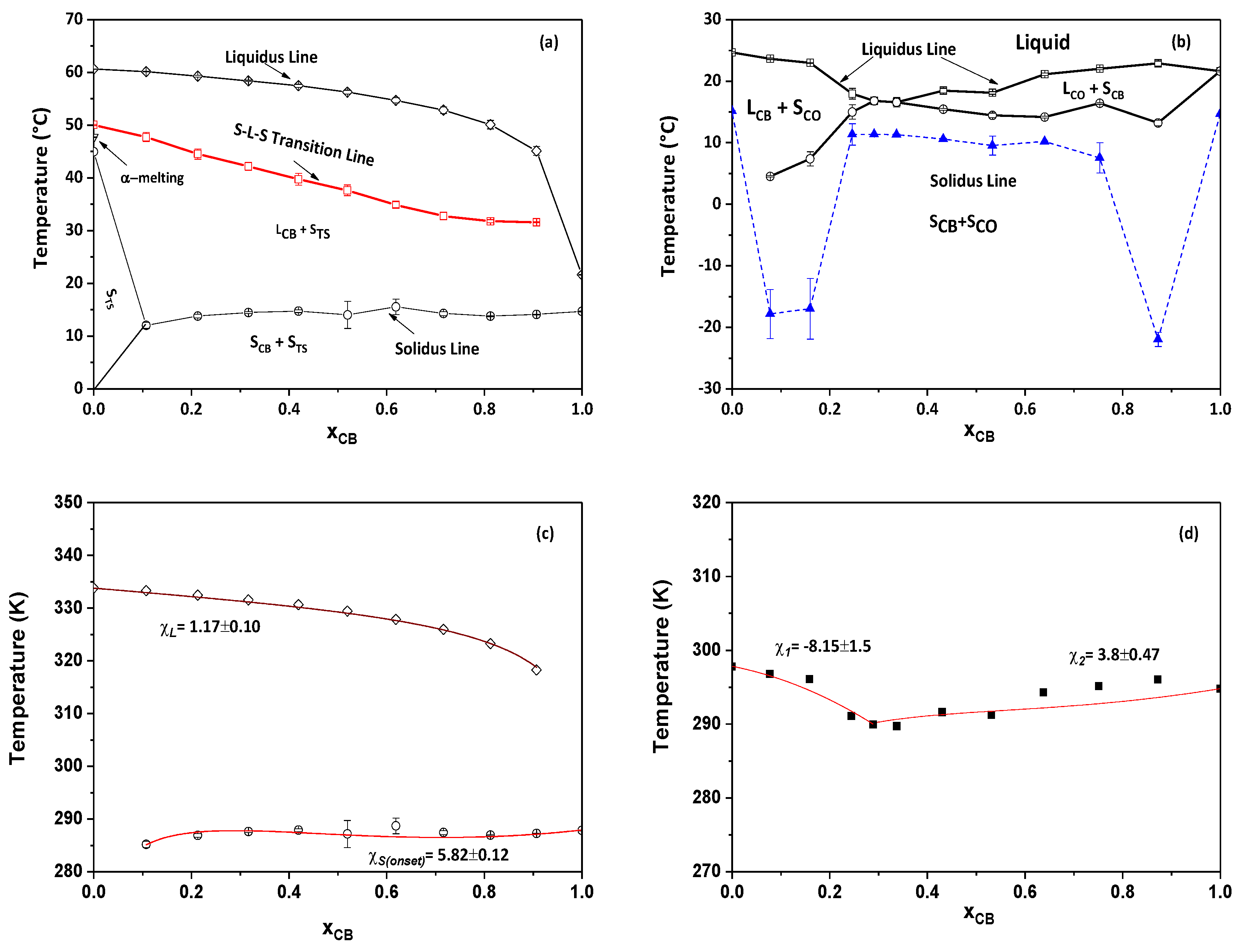
The polymorphism and phase transitions of cocoa butter (CB) have been reexamined separately by differential scanning calorimetry (DSC) and X-ray diffraction as a function of temperature (XRDT) at scanning rates between 0.1 to 5°C/min and 0.1 to 2°C/min, respectively. A new instrument, which allowed simultaneous DSC and XRDT recordings from the same sample by taking advantage of the high ...
Phase diagram for chocolate
Controlling phase/state transitions in sugar-based foods like confections requires knowledge of equilibrium considerations and kinetic effects. The equilibrium conditions, as described by the phase diagram, define what phase/state to expect if the food product attains phase equilibrium. Chocolate Experiment Science concepts you will learn about in these experiments: 1. Phase diagrams and melting point 2. Polymorphisms 3. Hydrophilicity vs. hydrophobicity and emulsion 4. Diffraction of light Safety: Adult supervision needed for any heating of chocolate! Background: Chocolate is probably one of the most popular food types in the ... pure chocolate (13 phase). The eutectic is, of course, chocolate ripple. An interesting feature of the diagram is the sloping solvus line. When a composition within the proper range is allowed to thaw slightly, into the α region (a solid solution of chocolate in vanilla), and is then cooled to a
Phase diagram for chocolate. Title: The Science of Chocolate: Interactive Activities on Phase Transitions, Emulsification, and Nucleation Authors: Amy C. Rowat, Kathryn A. Hollar, Howard A. Stone, and Daniel Rosenberg Journal: Journal of Chemical Education, 2011, 88 (1), pp 29-33 From our free online course, "Science & Cooking: From Haute Cuisine to Soft Matter Science (chemistry)": https://www.edx.org/course/science-and-cooking-chemi... motes a continuous fat phase that evenly coats the sugar and cocoa solids thus producing a flowable liquid. The final processing steps are tempering and solidification of the confectionery product. Immediately after tempering, the chocolate is molded and cooled to approximately 16°C for proper contraction. The phase diagram shown below comes from the University of Cambridge website. For those familiar with the terms, it is a so called practical phase diagram. Phase diagram of sucrose and water. Source. Since we are talking ice cream here, we'll only discuss one region. Take a sugar solution with 20% sucrose at -18°C, we end up in the 'ice ...
Nearly everyone loves chocolate, which makes this an excellent topic for communicating scientific concepts to the general public and to students in the classroom. Here we present the outline and activities for an interactive presentation on the science of chocolate for nonspecialists and their children ages 6 and up. We design the presentation around three major questions related to observable ... The process thus is as follows [4]: 1. Bring chocolate up to 45°C to melt all six phases of chocolate. 2. Cool chocolate to 27°C to allow crystals of phase IV and V to form. This step is accompanied by agitation to create seed crystals of these phases. 3. Heat up chocolate to 31°C to melt all phase IV crystals. 4. Chocolate production is a great example of materials science in practice, and the event explored topics such as the microscopic structure of chocolate, how phase instabilities of cocoa butter leads to fat and sugar blooms, and the impact of cocoa particle size on viscosity. However, there is more to chocolate making than just science, and the ... The form of the Clapeyron equation most often used is dP/dT = Δ S/ Δ V This equations states that the slope (rise/run) of an univariant equilibrium plotted on a P-T diagram is equal to the entropy change (Δ S) of the reaction divided by the volume change (Δ V) of the reaction.Using the Clapeyron equation, only the slope of the equilibrium is determined, not the actual position of the ...
But even when tempering is complete, it doesn't always mean the end of chocolate's phase changing. Form VI chocolate, while dull, waxy and slow to melt in the mouth, is actually more stable than ... Freeze Drying Definition. Lyophilisation or freeze drying is a process in which water is frozen followed by its removal from the sample initially by sublimation (primary drying) and then by sublimation (desorption).Since heat is not applied in this case.So,both nutritional qualities and sensory characteristics were better retained.Freeze drying ... The chocolate in Peanut Butter Cups is actually a solid solution containing some peanut butter. To confirm this simply do a taste test between M & M's vs. Reese's Pieces. This explains why phase Alpha on the diagram is not a line phase. Mostly, all the instructor has to do is go through the four transparencies with the class. They are self ... The binary eutectic phase diagram explains the chemical behavior of two immiscible (unmixable) crystals from a completely miscible (mixable) melt, such as olivine and pyroxene, or pyroxene and Ca plagioclase. Here we are going to generalize to two minerals, A and B, or P and Q. We want to observe the behavior of this system under two conditions ...

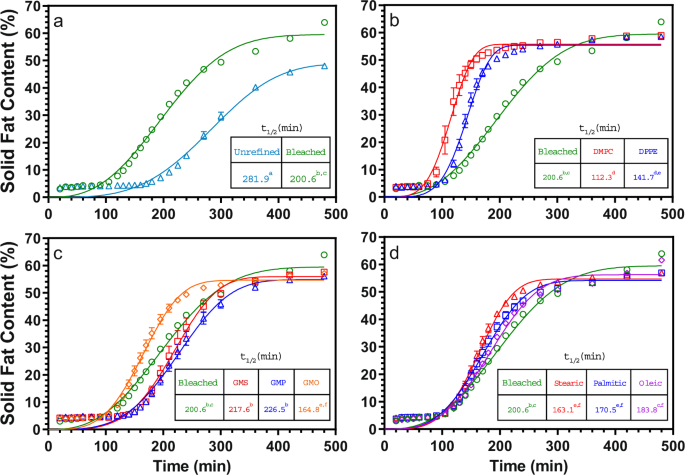
Foods Free Full Text Comparative Study On Mixing Behavior Of Binary Mixtures Of Cocoa Butter Tristearin Cb Ts And Cocoa Butter Coconut Oil Cb Co Html
represented on a phase diagram. • Boundaries of the phase diagram are determined by when the interaction energy, U, between molecules in food is equal to the thermal energy, k BT, times a con-stant, C. • Ice cream. Prepare ice cream using a slurry of salt water and ice to cool the base below 0 °C. • Use a phase diagram to explain how
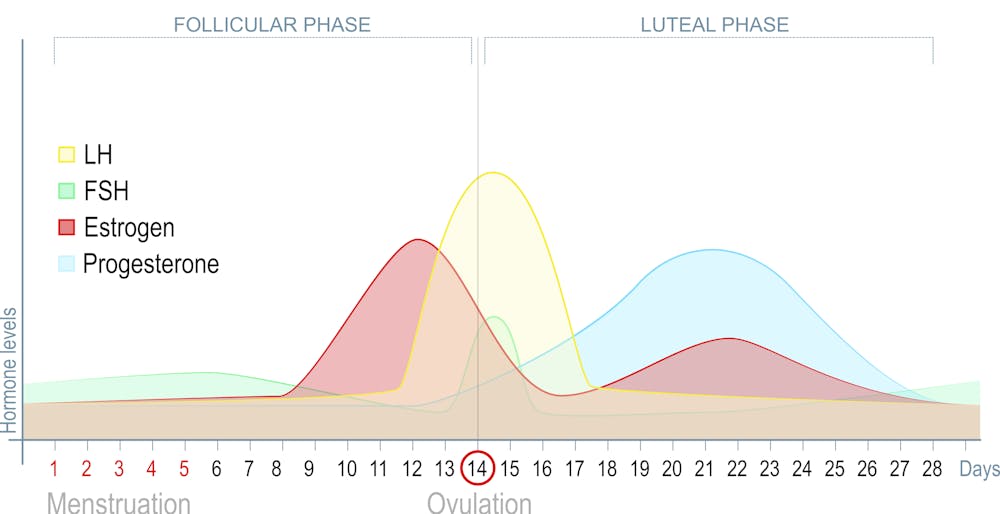
Diagram of a textbook menstrual cycle ("textbook" refers to how they lay it out as 28 days - women have all different lengths of a menstrual cycle that are normal) that shows the phases from the follicular phase (also known as the low hormone phase) through ovulation and into the luteal phase (also known as the high hormone phase.
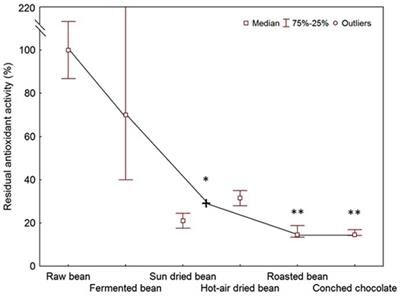
Frontiers From Cocoa To Chocolate The Impact Of Processing On In Vitro Antioxidant Activity And The Effects Of Chocolate On Antioxidant Markers In Vivo Immunology
MS15a, Gibbs Free Energy and Phase Diagrams 11/00 . The system can, in fact, lower its free energy even further by splitting up into a solid of composition X. S B. and a liquid of composition X. L B (shown on both diagrams). The gibbs free energy of the solid is given by point (4) on the g(X. B) diagram and that of the liquid by point (5) on ...

Solved Chocolate Is An Interesting Example Of Constantly Changing Mixture That Combines Many Different Concepts From Class And Prior Experiences These Include But Are Not Limited To Properties Of Mixtures Phase Diagrams Of
Lesson Plan: Chocolate Changes. In this lesson, students will review what they know about the three states of matter (solid, liquid, and gas). They will perform a word sort about states of matter and discuss how substances can exist in more than one state of matter. Following this they will contribute to a KWL (Know, Want to know, Learned) chart.
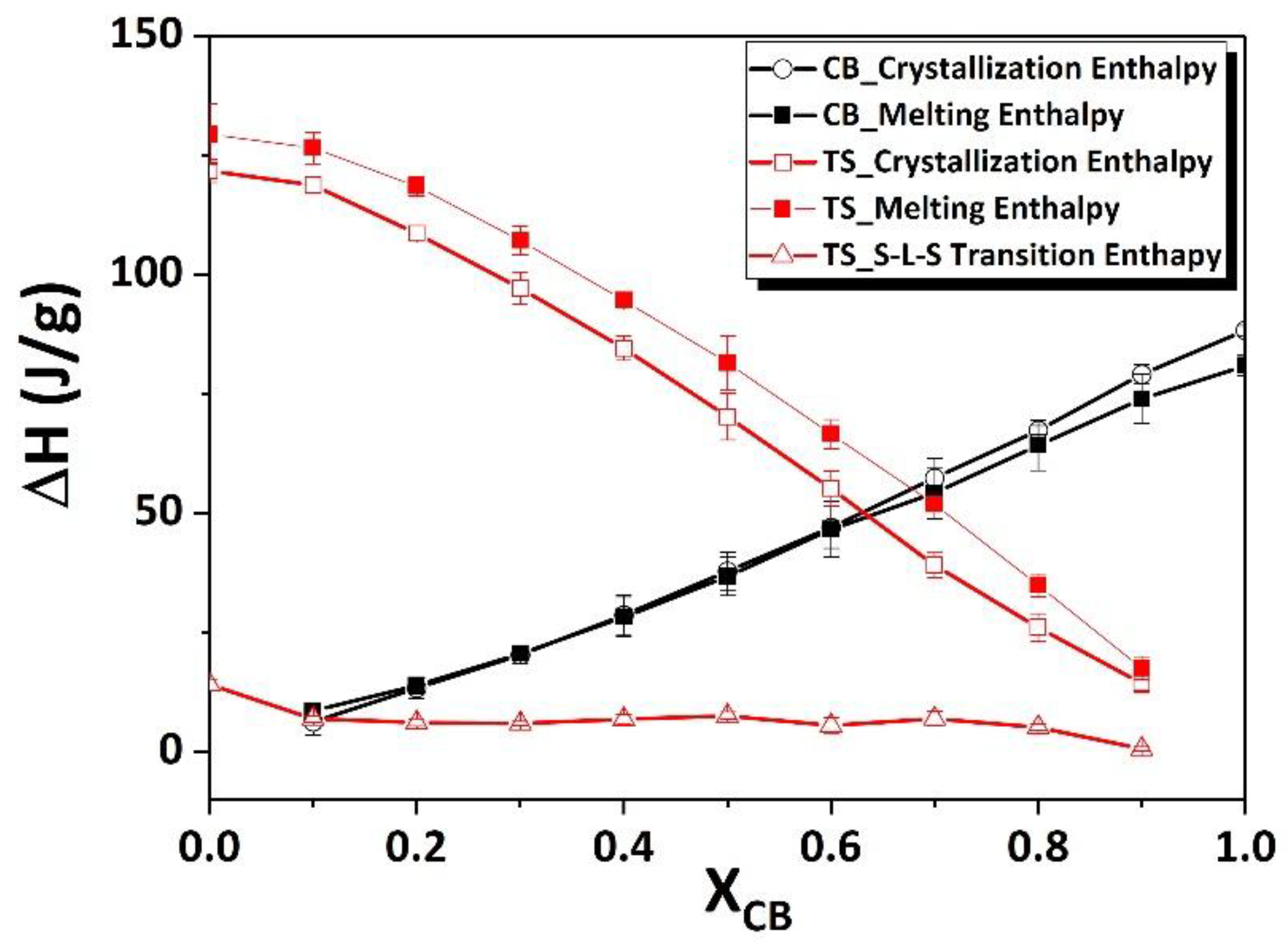
Foods Free Full Text Comparative Study On Mixing Behavior Of Binary Mixtures Of Cocoa Butter Tristearin Cb Ts And Cocoa Butter Coconut Oil Cb Co Html
Princeton University
Milk chocolate melts at a lower temperature than dark chocolate Melting temperature depends on material composition and on the shape of fat molecules; cocoa butter melts at around your body temperature Phases of matter, phase transitions, lipid composition Why does chocolate feel smooth? European-style chocolate versus Mexican-style chocolate
Interesting Facts About Chocolate In 11 Diagrams - LifeTitude says: September 22, 2015 at 3:52 am […] you, try and make some simple treats at home. Often, working with chocolate requires you to learn tempering, which is a way to rearrange the cocoa solids and make the chocolate more shiny and easy to […]
Exploring The Polymorphic Structures In Milk Chocolate Dark Chocolate And Cocoa Butter Physics Of Cooking
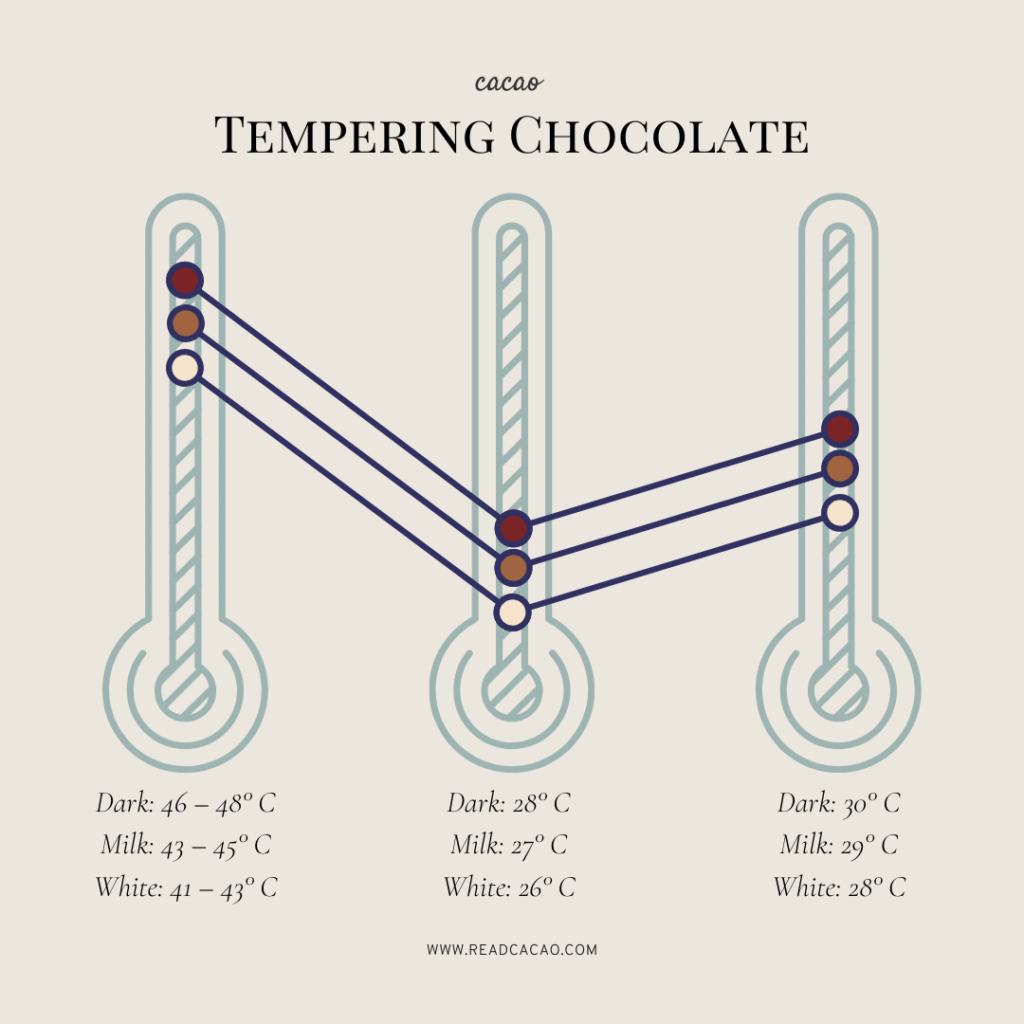
When tempering chocolate, it is the crystal structure of the cocoa butter that chocolatiers are manipulating. 'Cocoa butter is a six-phase polymorphic crystal,' explains chocolatier ...
chocolate. Google. "cocoa butter" "phase diagram" 300 hits. Chocolate is in essence cocoa mass and sugar particles suspended in a. cocoa butter matrix. Cocoa butter is a mixture of triglycerides in. which stearoyl, oleoyl and palmitoyl groups predominate. It is the. polymorphs of this matrix that influence the quality of chocolate.
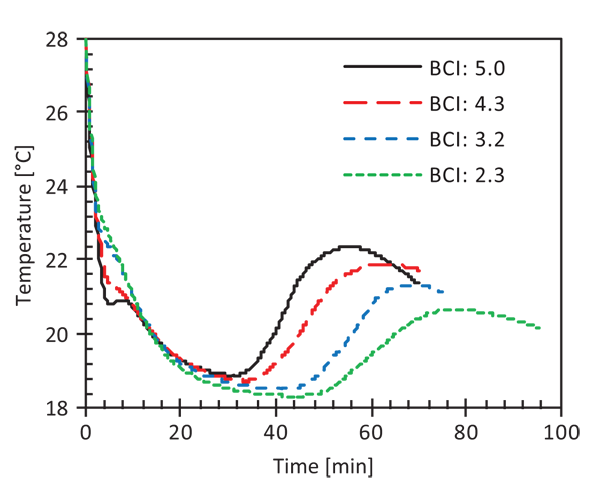
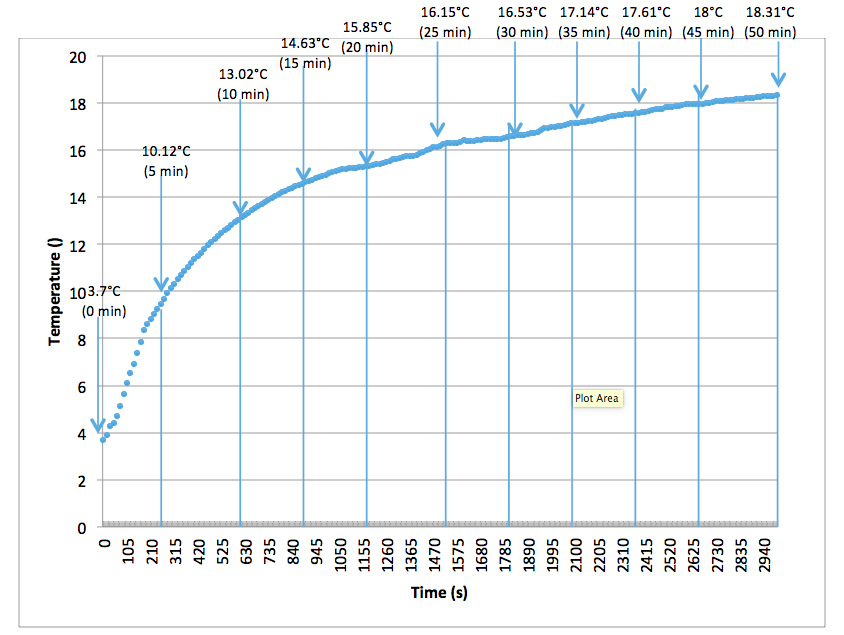
Inform Magazine February 2021 Exothermal Analysis Of Cocoa Butter Cocoa Liquor And Chocolate Mass With Multitherm Tc
Crystallization of cocoa-butter in the β phase from the melt under static conditions is only possible using the memory effect of cocoa-butter. Under all other conditions polymorphs with lower melting temperatures develop, whereas the β phase is the preferred one in chocolate. SAXS experiments proved 1,3-distearoyl-2-oleoylglycerol seeds with triple chain-length packing initiate the β ...
PHASE DIAGRAM OF CHOCOLATE AND VANILLA. Phase diagram of chocolate and vanilla. How much vanilla will chocolate take? Guesses? Can you use the Lever Rule? (Source: Kenneth A. Jackson at the University of Arizona. Courtesy of NASA) [+ more] Labels: molecular gastronomy.
pure chocolate (13 phase). The eutectic is, of course, chocolate ripple. An interesting feature of the diagram is the sloping solvus line. When a composition within the proper range is allowed to thaw slightly, into the α region (a solid solution of chocolate in vanilla), and is then cooled to a
Chocolate Experiment Science concepts you will learn about in these experiments: 1. Phase diagrams and melting point 2. Polymorphisms 3. Hydrophilicity vs. hydrophobicity and emulsion 4. Diffraction of light Safety: Adult supervision needed for any heating of chocolate! Background: Chocolate is probably one of the most popular food types in the ...
Controlling phase/state transitions in sugar-based foods like confections requires knowledge of equilibrium considerations and kinetic effects. The equilibrium conditions, as described by the phase diagram, define what phase/state to expect if the food product attains phase equilibrium.

Crystallisation In Water In Cocoa Butter Emulsions Role Of The Dispersed Phase On Fat Crystallisation And Polymorphic Transition Sciencedirect
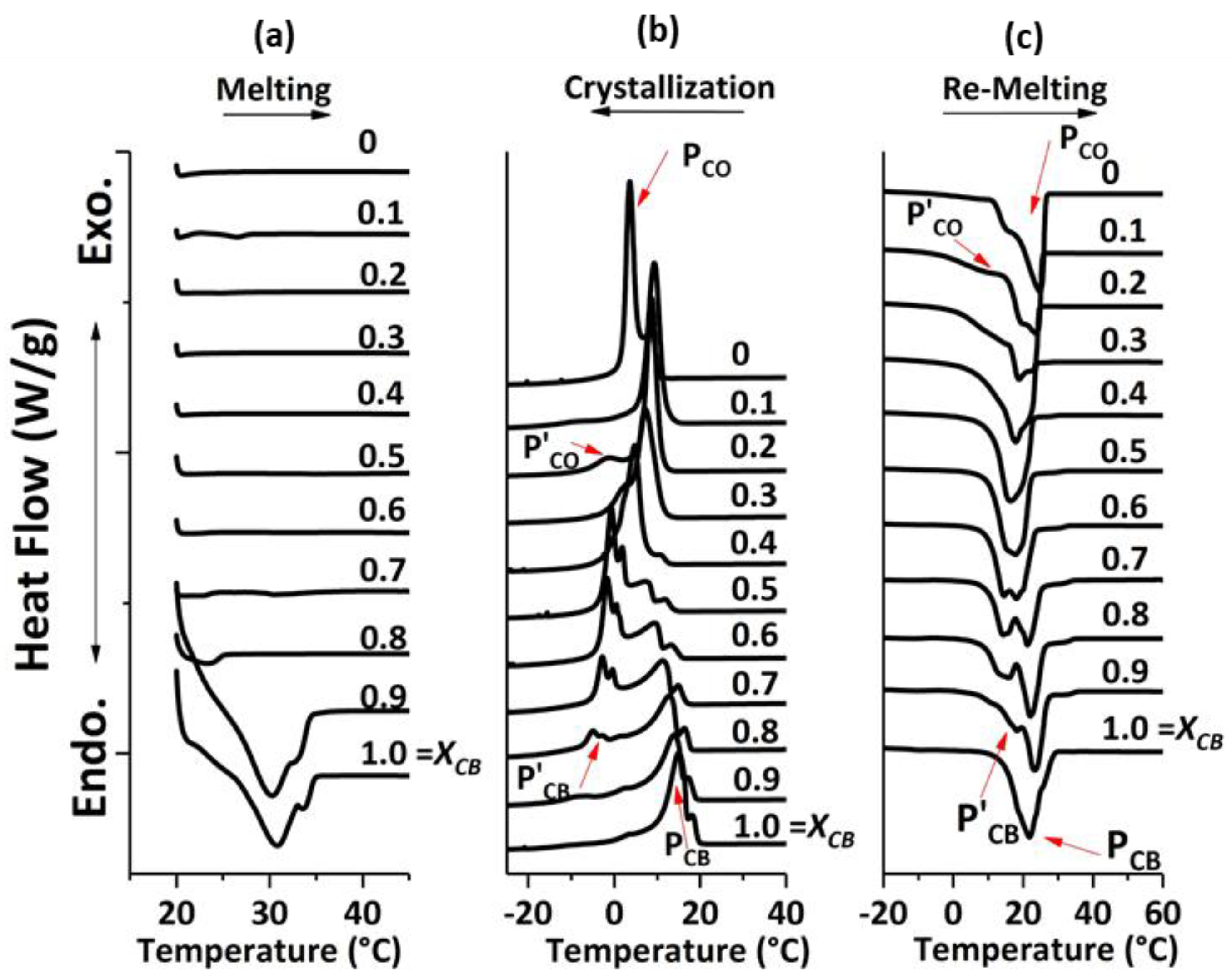
Foods Free Full Text Comparative Study On Mixing Behavior Of Binary Mixtures Of Cocoa Butter Tristearin Cb Ts And Cocoa Butter Coconut Oil Cb Co Html

Tuning Chocolate Flavor Through Development Of Thermotolerant Saccharomyces Cerevisiae Starter Cultures With Increased Acetate Ester Production Applied And Environmental Microbiology

Oleuropein Enriched Chocolate By Extra Virgin Olive Oil Blunts Hyperglycaemia In Diabetic Patients Results From A One Time 2 Hour Post Prandial Cross Over Study Clinical Nutrition
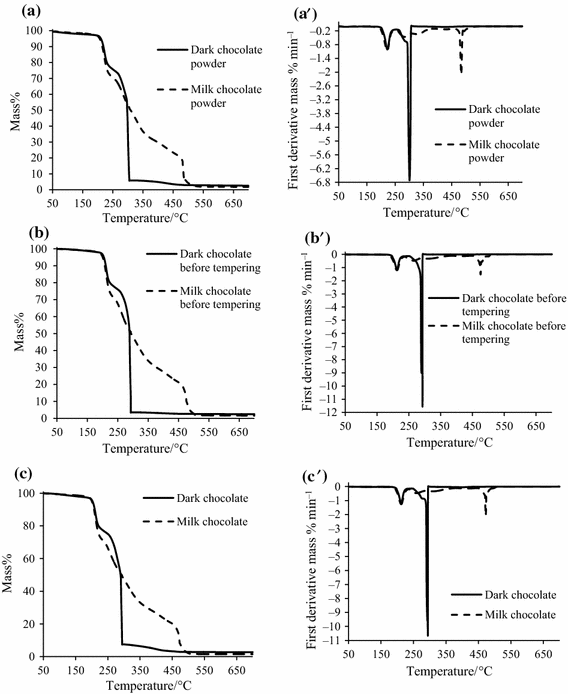
Thermogravimetric Characterization Of Dark And Milk Chocolates At Different Processing Stages Springerlink

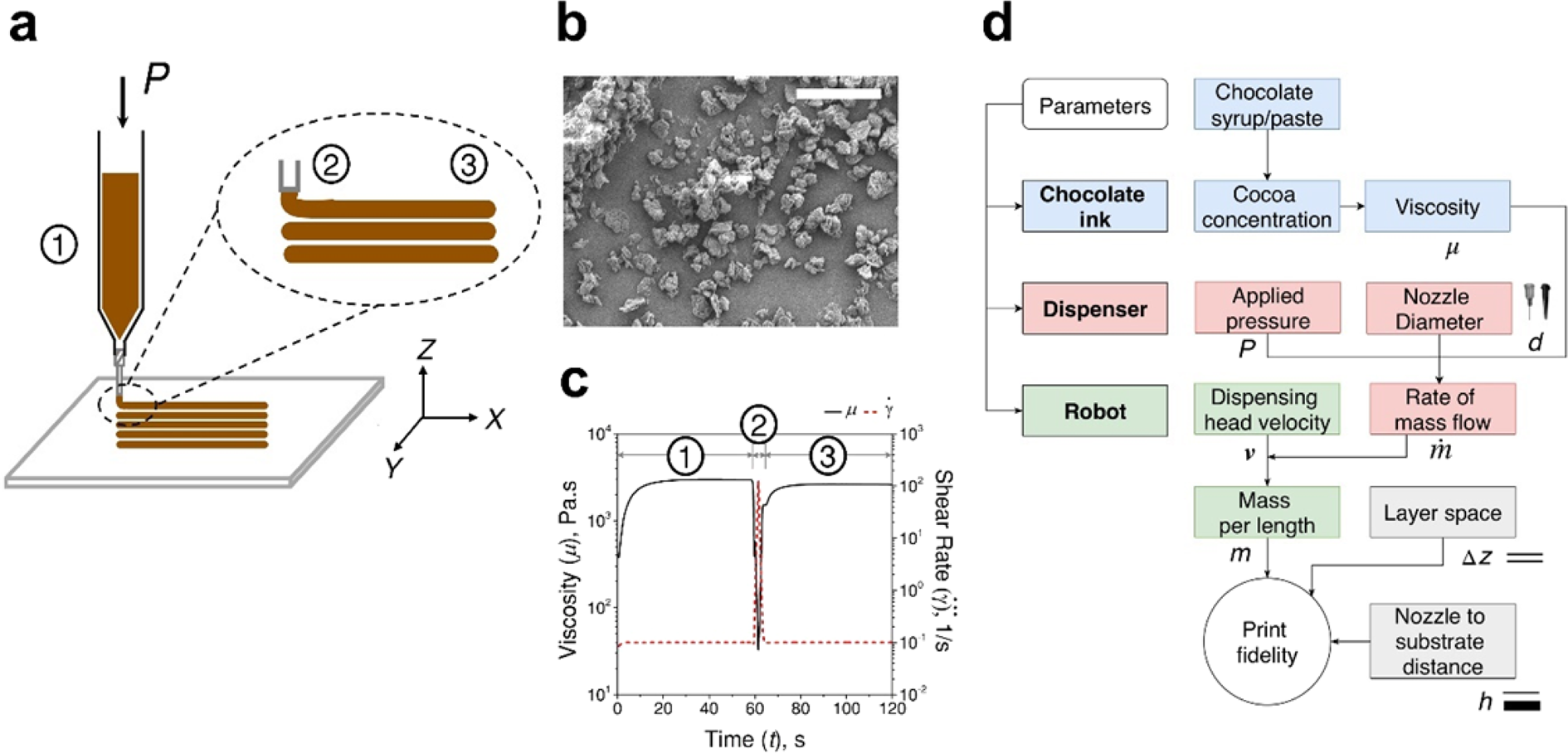
Conching Chocolate Is A Prototypical Transition From Frictionally Jammed Solid To Flowable Suspension With Maximal Solid Content Pnas

Physical Chemical Properties Of Shea Cocoa Butter Blends And Their Potential For Chocolate Manufacture Rodriguez Negrette 2019 Journal Of The American Oil Chemists Society Wiley Online Library
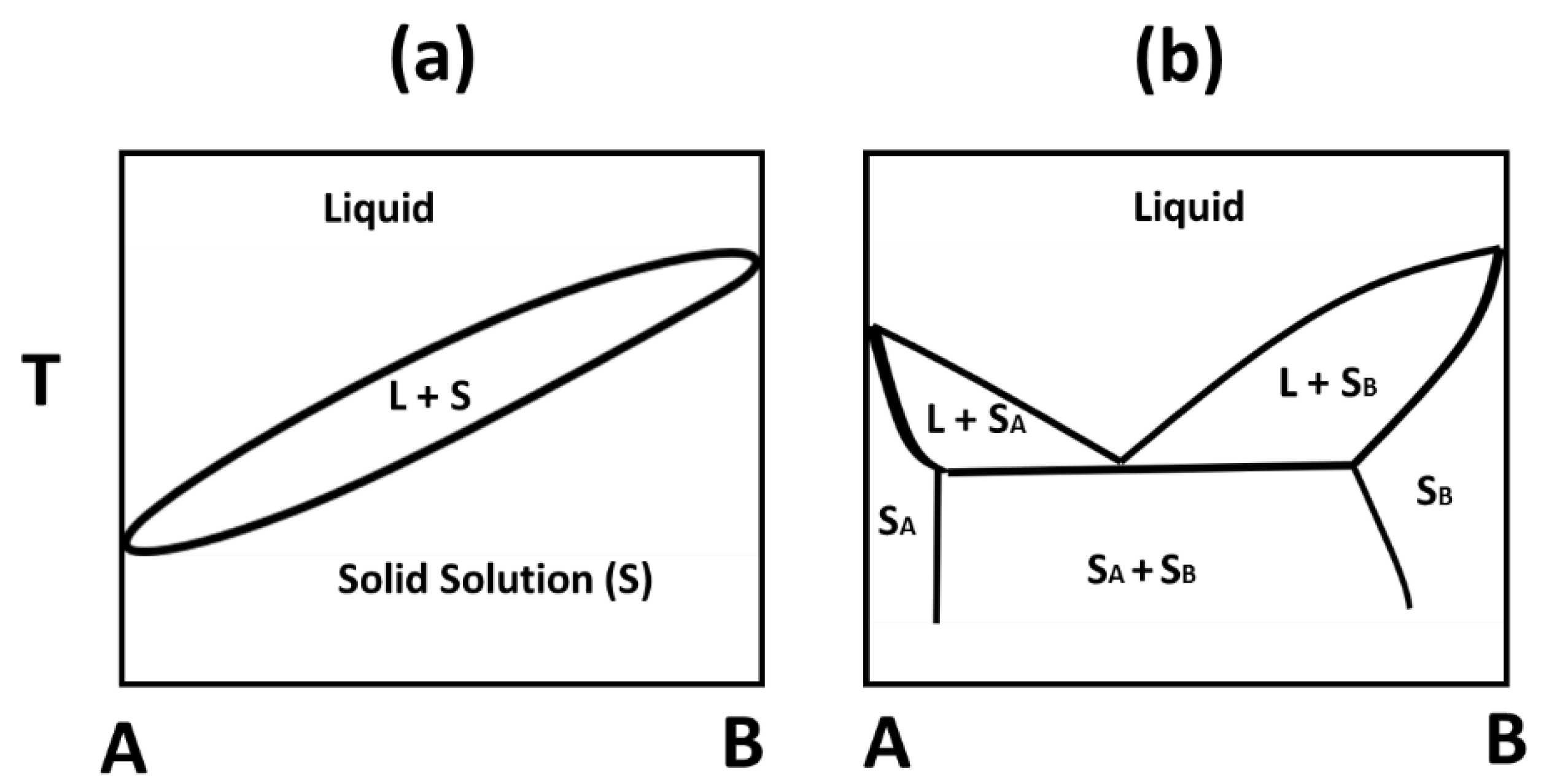
Foods Free Full Text Comparative Study On Mixing Behavior Of Binary Mixtures Of Cocoa Butter Tristearin Cb Ts And Cocoa Butter Coconut Oil Cb Co Html
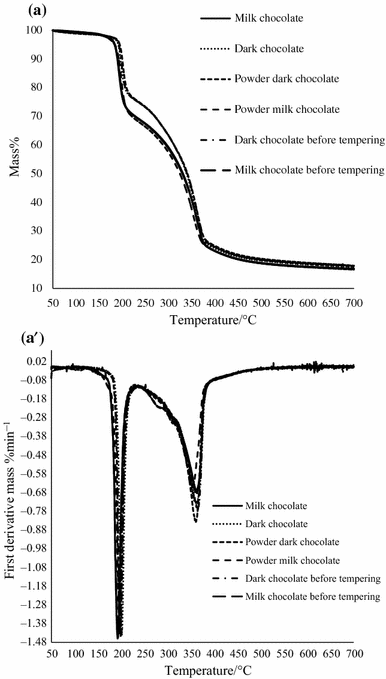
Thermogravimetric Characterization Of Dark And Milk Chocolates At Different Processing Stages Springerlink

Dark Chocolate Intake Positively Modulates Redox Status And Markers Of Muscular Damage In Elite Football Athletes A Randomized Controlled Study









.png)




Comments
Post a Comment