43 co molecular diagram
Molecular orbitals diagrams of [Co(NH3)6]3+ Estimation of oxalic acid experimental data. Mithil Fal Desai. Polarisation and polarisability. Mithil Fal Desai. Evidences for covalent bonding in complexes. Mithil Fal Desai. Molecular orbitals diagrams of [Ti (H2O)6]3+. Mithil Fal Desai. Molecular orbitals diagrams without pi interactions. techiescientist.com › co-lewis-structureCO Lewis Structure, Geometry, and Hybridization - Techiescientist 1 day ago · Lewis structure of single carbon and oxygen atom separately is as shown below. The atomic number of the carbon is six which makes its electronic configuration 1s2 2s2 2p2. As the 2p shell has a capacity of holding up to six electrons, there comes a deficiency of four electrons. So, carbon has four valence electrons which are ready to act in a bond formation to stable its atomic structure.
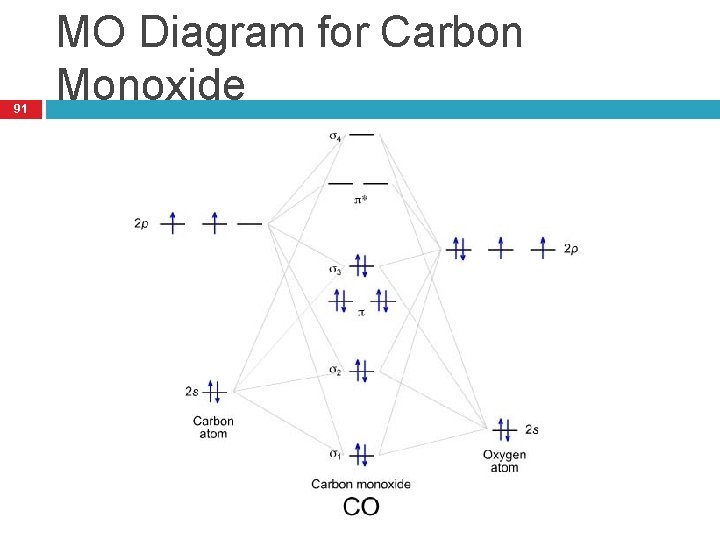
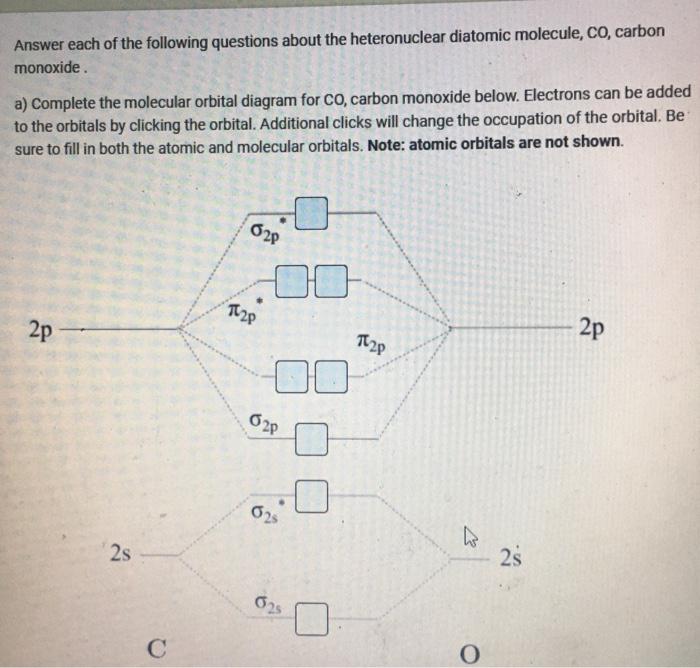
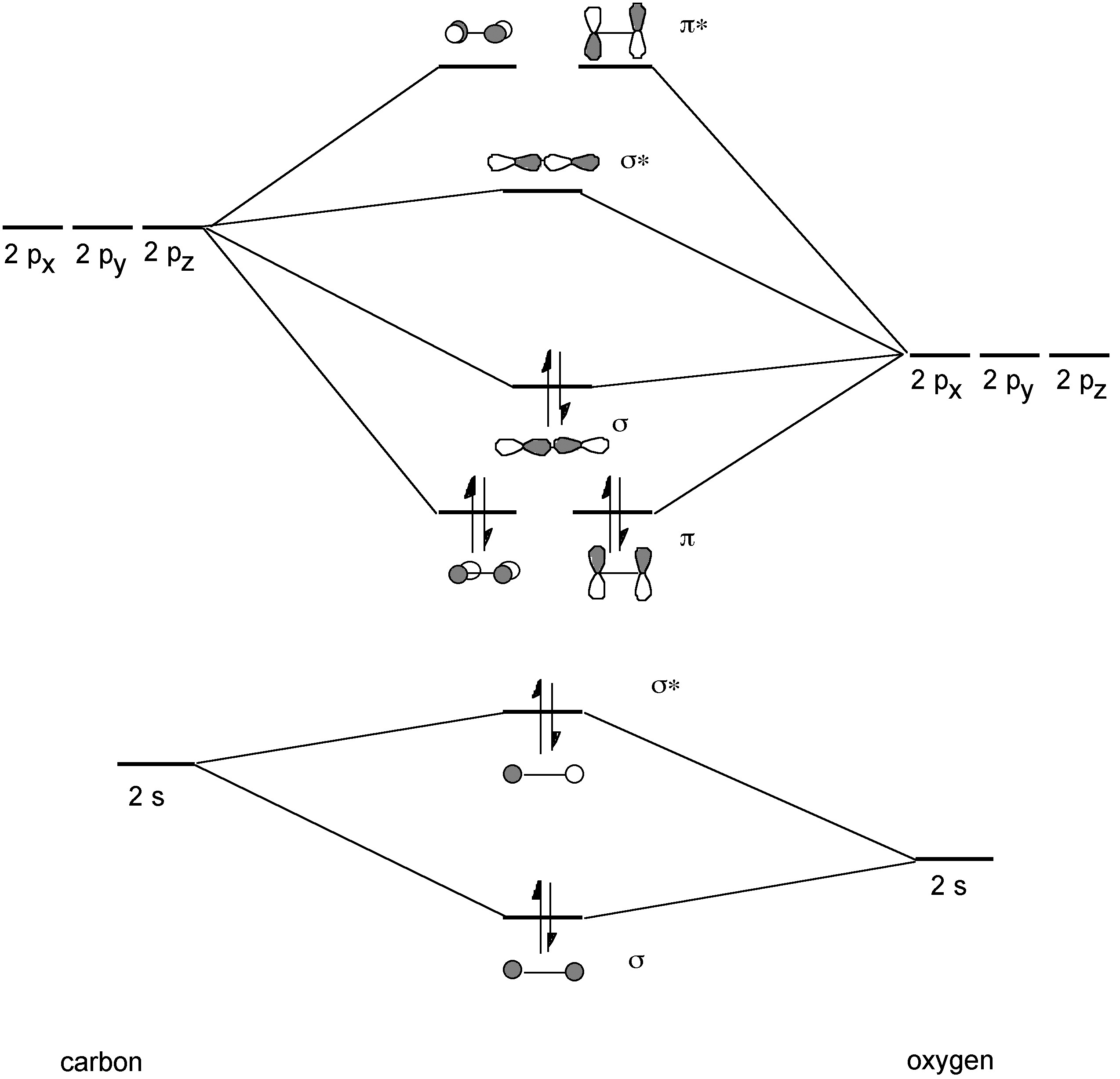
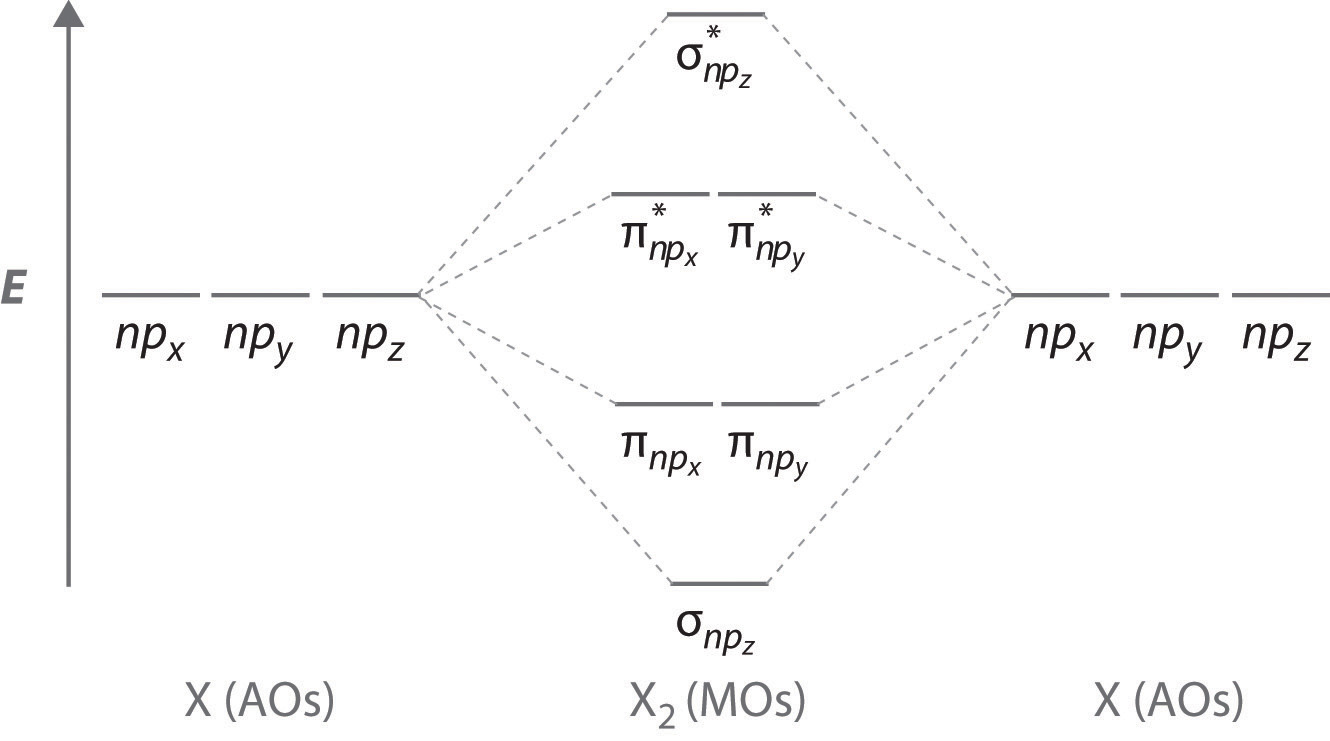
How Do You Read A Molecular Orbital Diagram? In the molecular orbital (MO) diagram, the individual atomic orbitals (AO) are on the far left for C and far-right for O. The MOs of CO are formed due to the overlapping of the AOs. There are either or π orbitals. When two s AOs overlap, there are one bonding and one antibonding MOs that is MO and * MO.
Co molecular diagram
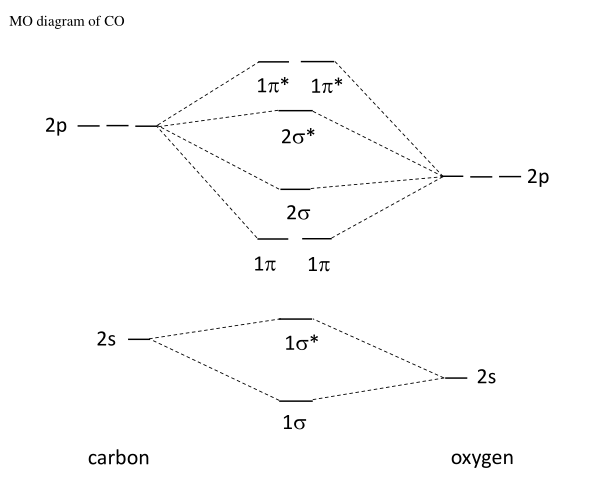
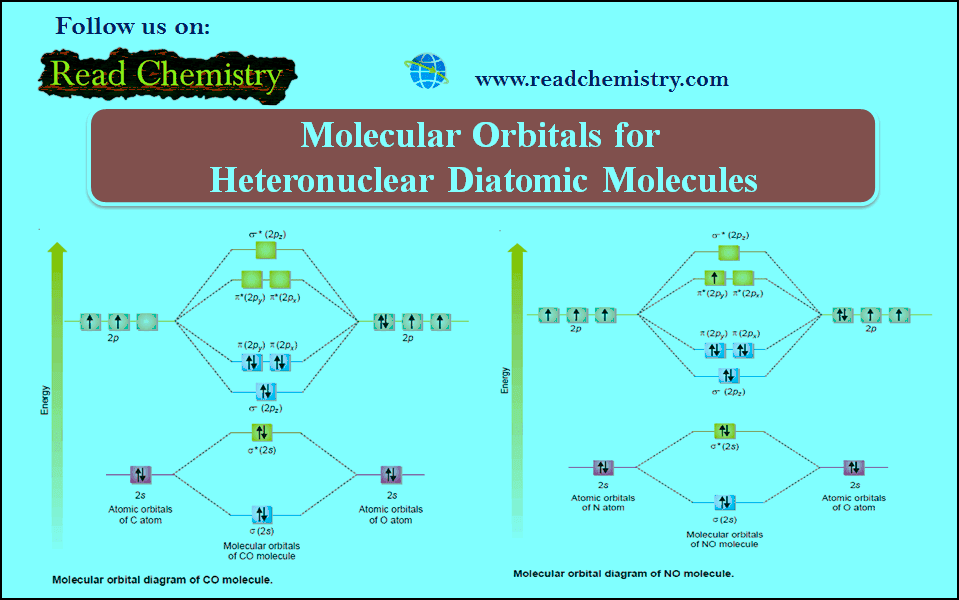
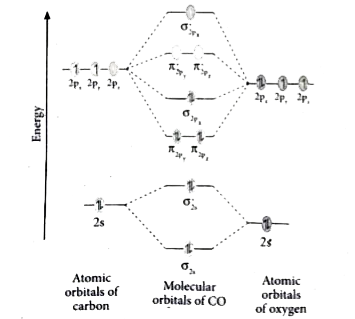
Molecular orbital diagram of CO and NO Molecular orbital diagram of CO and NO 1. ENERGY C 2s 2p atomic orbital O 2s 2p atomic orbital 4σ CO 2σ 1σ 3σ 2𝚷 1𝚷 molecular orbital C (Z=6) [He], 2s2,2p2 O (Z=8) [He], 2s2,2p4 Molecular energy diagram for carbon monoxide (CO) 2. ENERGY O 2s 2p N 2s 2p atomic orbital atomic orbital NO 2σ 1σ 3σ 4σ 2𝚷 1𝚷 molecular orbital N ... Heteronuclear Molecules Energy Level Diagram of CO Energy Level Diagram of CO ... N2 triple is very strong (only the CO triple bond is stronger) ... Molecular Orbitals of Polyatomic Molecules.18 pages MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
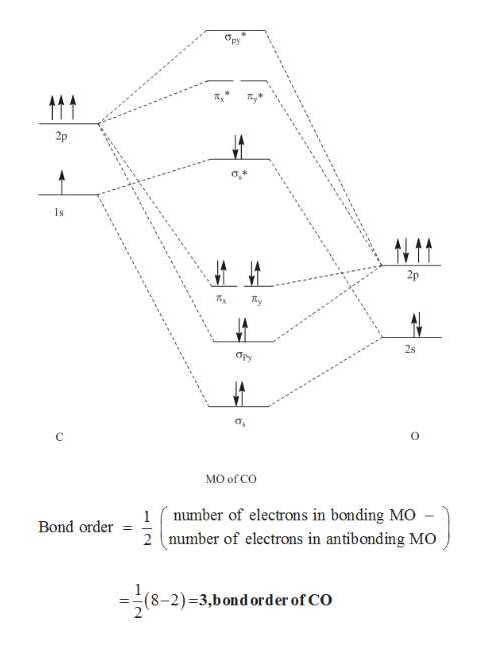
Co molecular diagram. What is the molecular orbital energy diagram of CO ... - Quora Molecular orbital energy level diagram of CO molecule can be given as. CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).According to molecular orbital diagram, molecular orbital configuration is given as σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰ Thus , bond order = 1/2 (8-2)=3 83.5K views View upvotes (i) It has a double bond Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ... schematron.org › carbon-monoxide-molecular-orbitalCarbon Monoxide Molecular Orbital Diagram Explanation May 09, 2018 · There are 4 electrons in the outer shell of carbon and 6.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Molecular Orbitals for Carbon Monoxide - Newcastle University CO is a very stable 10-valence-electron molecule, isoelectronic with [CN] - and with N 2, which has a slightly lower bond dissociation energy than CO The formal bond order of CO is 3, from about one σ- bond and two π- bonds Its most important property is burning in air to give CO 2 , in the combustion of fossil fuels
3.3.4: Assembling a complete MO diagram - Chemistry LibreTexts A molecular orbital interaction diagram shows how atomic or molecular orbitals combine together to make new orbitals. Sometimes, we may be interested in only the molecular orbital energy levels themselves, and not where they came from. A molecular orbital energy level diagram just shows the energy levels in the molecule. PDF Lecture 3 Figure 3-1 Molecular orbitals of Cr(CO) 6 (Only interactions between Ligand (σ- and π*) orbitals and metal d-orbitals are shown.) Simplified MO energy level diagram for Cr(CO) 6. Note the empty π* orbitals. Only three are involved in overlap with metal d orbitals. 5.3.1: Polar bonds - Chemistry LibreTexts Carbon monoxide MO diagram Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The valence molecular orbitals in both atoms are the 2 s and 2 p orbitals. The molecular orbital diagram for carbon monoxide (Figure 5.3.1. What is the molecular orbital energy diagram of CO? - Quora Jan 7, 2017 — CO is isoelectronic to N2 C and O are sp hybridized there is an Csp Osp sigma bond, 2Cp Op pi bonds the remaining sp orbitals each has an unshared pair of ...4 answers · 30 votes: First let us know what molecular orbital diagram is: A molecular orbital diagram, or MO diagram, ...What is the bond order of CO?15 answersSep 16, 2016When we draw an MO diagram of CO or CO2, why is ...3 answersSep 7, 2020More results from
Solved Starting from the t2g -eg* splitting of [Co(H2O)6]3 ... Fill in the d-electrons on each MO diagram. *** Please, show with details how to solve the problem step by step *** Question: Starting from the t2g -eg* splitting of [Co(H2O)6]3+ show how its molecular orbital (MO) diagram differs from that of [CoF6]-3. Fill in the d-electrons on each MO diagram. Molecular orbital diagram Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Molecular orbital theory. Heteronuclear ... - YouTube 12-12 This video describes the molecular orbital theory diagram of CO, placing emphasis on how MO theory differs for homo and heteronuclear diatomics geometryofmolecules.com › co-lewis-structureCO Lewis structure, Hybridization, and Molecular Geometry ... CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide) Carbon Monoxide is a colorless and odorless gas. This gas is less dense than the air and flammable. People know about this gas, as it can also cause poisoning. Carbon Monoxide is a toxic gas that binds with hemoglobin, which interferes with its binding with Oxygen.
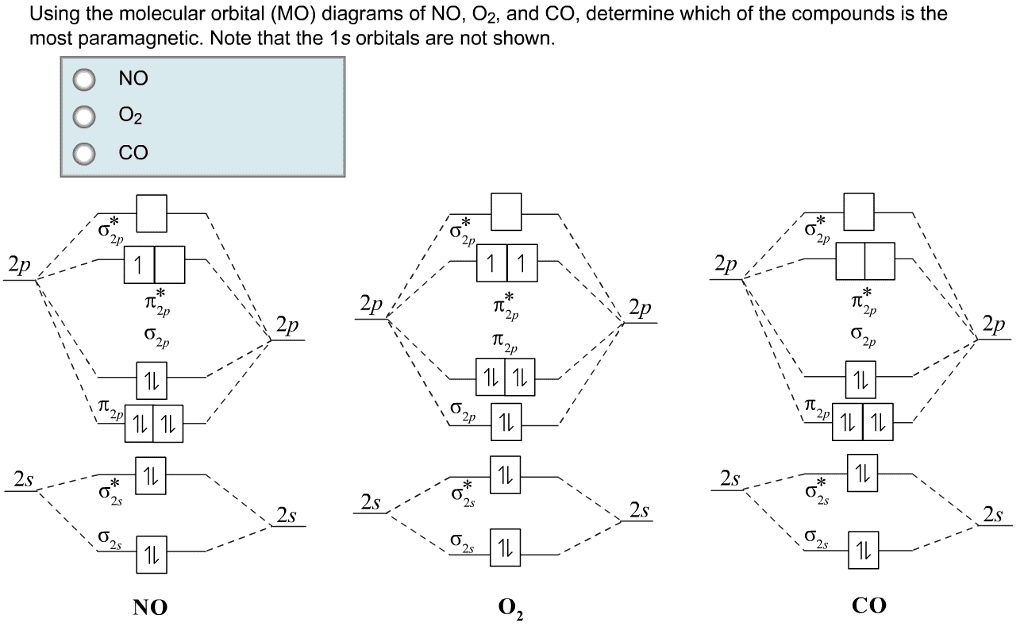
By writing molecular orbital configuration for NO,CO,O2 ... Mar 18, 2018 · "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram.
inorganic chemistry - Stack Exchange Particularly, from what I read the reason we envision carbon as being partially negatively charged in CO is that the HOMO of the molecule lies closer to the carbon orbitals. However, from the MO diagram, it can be seen that the p-oxygen orbitals are closer to the HOMO than both of the orbitals of carbon.
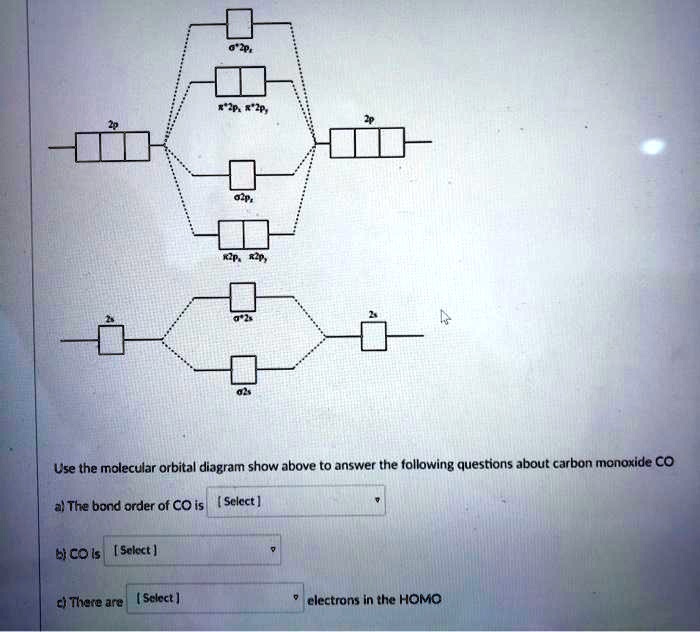
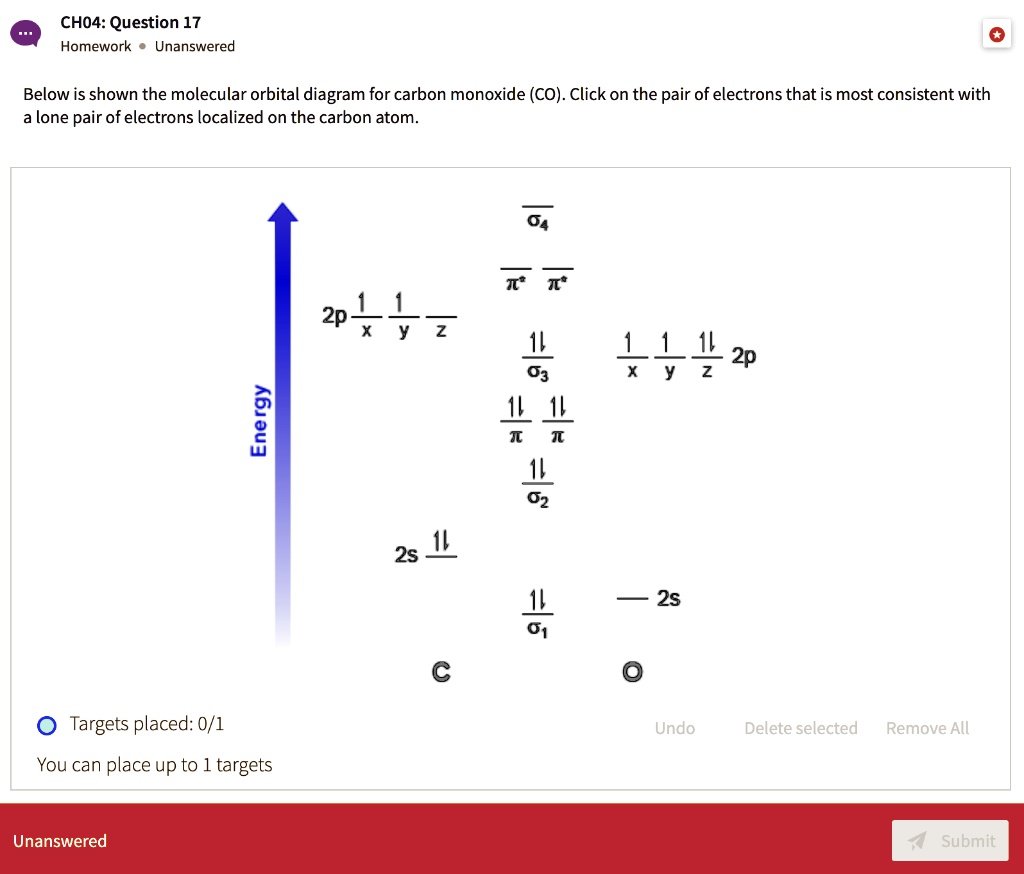
Solved SLO 3.1 Molecular orbital theory (a) (Comprehension ... Draw the molecular orbital diagram for CO (CO anion) with all of its corresponding electrons. Remember that the MO diagram of CO follows the orbital pattern associated with N2. Relative energies of MOs with respect to the AOs matters here! b. Label your diagram clearly with the respective symmetries of each orbital and whether they are bonding ...
wiringall.com › carbon-monoxide-molecular-orbitalCarbon Monoxide Molecular Orbital Diagram Explanation A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. . combinations such as CO and NO show that the 3σg MO is higher in energy. Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals.
Draw MO diagram of CO and calculate its bond ... - Sarthaks Electronic configuration of CO molecule is: σ1s2 σ*1s2 σ2s2 σ*2s2 π2py2 π2pz2 π2px2 3. Bond order = = \(\frac{N_b-N_a}{2}\) = \(\frac{10-4}{2}\) = 3 4. Molecule has no unpaired electron, hence it is diamagnetic. Please log inor registerto add a comment. ← Prev QuestionNext Question → Find MCQs & Mock Test Free JEE Main Mock Test
Heterodiatomic Molecules: MO diagram for CO - GRAYLARK Example: CO This diagram is based on calculations and comparison to (But it is not drawn exactly, just approximately.) about the sp-mixing and the shapes and sizes of MOs. Notice that the bonding orbitals are bigger on the oxygen and the antibonding orbitals are bigger on the carbon. We will talk more about the consequences of this later.
8 - Drawing Molecular Orbital Diagrams Firstly, we now add our newly acquired antibonding molecular orbitals. There are now four types of orbitals to concern ourselves with: (1) sigma bonding, (2) sigma antibonding, (3) pi bonding, and (4) pi antibonding. The way these bonds are placed on any molecular orbital diagram is according to how the atomic orbitals that make the MOs mix.
MOT of CO+ molecule | Tricks For Molecular Orbital Theory ... This video for molecular orbital theory (MOT) in chemical bonding for CO+ molecule is by VIKAS MALI sir . Energy diagram of COshort tricks for Chemistry eve...
Draw MO diagram of CO and calculate its bond ... - Shaalaa Molecular orbital diagram of Carbon monoxide molecule (CO) Electronic configuration of C atom: 1s 2 2s 2 2p 2. Electronic configuration of O atom: 1s 2 2s 2 2p 4. Electronic configuration of CO molecule : σ 1s2, σ 1s*2, σ 2s2, σ 2s*2, π 2py2, π 2pz2 σ 2px2. Bond order = N b N a N b - N a 2. = 10 - 4 2 = 3.
› molecular-orbitalMolecular Orbital Diagram of CO - All About Chemistry MO Diagrams; NEET; Molecular Orbital Diagram of CO. By. All About Chemistry - July 2, 2020. 1. 269. Molecular Orbital Diagram of CO. TAGS; Molecular Orbital Diagram; Previous article Wohl-Ziegler Bromination. Next article Molecular Orbital Diagram of NO. All About Chemistry. .
orbitals - Chemistry Stack Exchange Point out key differences between the diagrams and use the diagram to explain why $\ce{CO}$ acts as a two-electron donor through carbon rather than through oxygen. Understandably, the key difference between these molecules is that $\ce{CO}$ is heteronuclear, and thus will have differences in energy between the molecular orbital and the atoms.
how to draw molecular orbital diagram of co CONTROLS Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Draw the MO diagram for B_2. E All of these are false. Determine point group of molecule if linear use D2hand C2vinstead of Dhor Cv 2. Bond order bonding electrons - antibonding electrons 2.
MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Heteronuclear Molecules Energy Level Diagram of CO Energy Level Diagram of CO ... N2 triple is very strong (only the CO triple bond is stronger) ... Molecular Orbitals of Polyatomic Molecules.18 pages
Molecular orbital diagram of CO and NO Molecular orbital diagram of CO and NO 1. ENERGY C 2s 2p atomic orbital O 2s 2p atomic orbital 4σ CO 2σ 1σ 3σ 2𝚷 1𝚷 molecular orbital C (Z=6) [He], 2s2,2p2 O (Z=8) [He], 2s2,2p4 Molecular energy diagram for carbon monoxide (CO) 2. ENERGY O 2s 2p N 2s 2p atomic orbital atomic orbital NO 2σ 1σ 3σ 4σ 2𝚷 1𝚷 molecular orbital N ...







![Molecular orbitals diagrams of [Co(NH3)6]3+](https://image.slidesharecdn.com/molecularorbitalsdiagramsofconh3631-211121141527/95/molecular-orbitals-diagrams-of-conh363-2-638.jpg?cb=1637504464)











Comments
Post a Comment